|
Home
The molecule Physical properties Chemical properties Antimicrobial properties Structure-activity relationships Biosynthesis Chemical syntheses References |
Structure-activity relationships
Analysis of the relationships between the molecular structures of tetracyclines and the
in vivo biological activities of these compounds led to conclude that the
characteristic chemotherapeutic activity of this group of antibiotics is dependent upon
the maintenance of all of the structural and stereochemical features of the following
expression [20]:
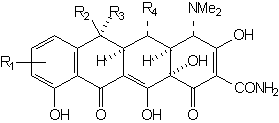
wherein the groups R1 - R4 are the only functions which may be varied without effecting a substantial decrease in antimicrobial activity. The simplest structure which embodies all of the elements necessary for activity is 6-demethyl-6-deoxytetracycline (sancycline):
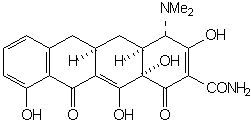
Functional groups at positions 5, 6, and 7 may be removed without drastically altering the antimicrobial properties. The right configuration at C-5a and C-4 is essential for activity. Equilibration involving C-4 leads to the relatively inactive 4-epi-tetracyclines (quatrimycins) [25]. The hypothesis has been advanced that the principal active center is the C(11), C(12) diketone system of rings B and C [26,27,8].