Aggarwal Group Research
Practical Guides



Research Overview
Lithiation-Borylation
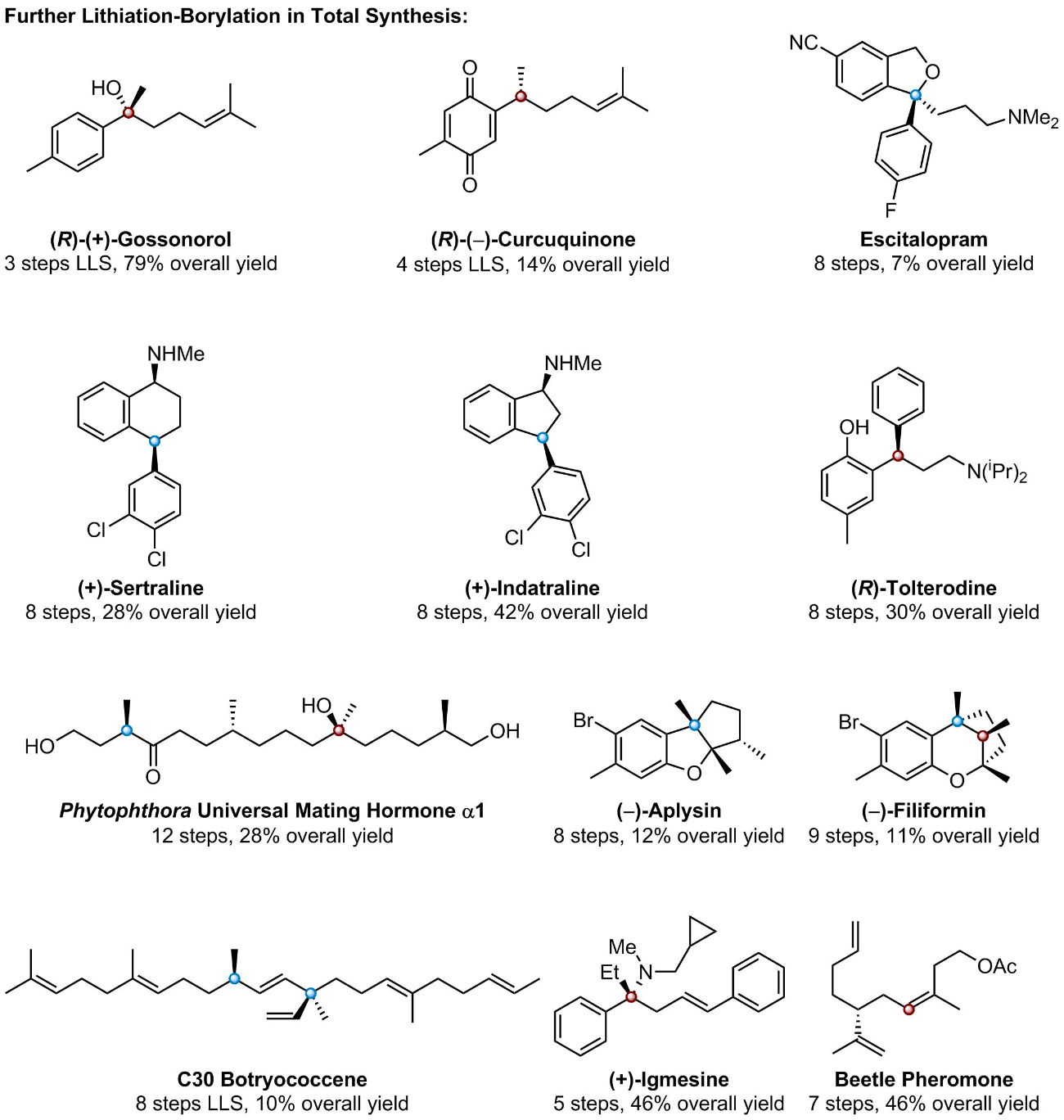
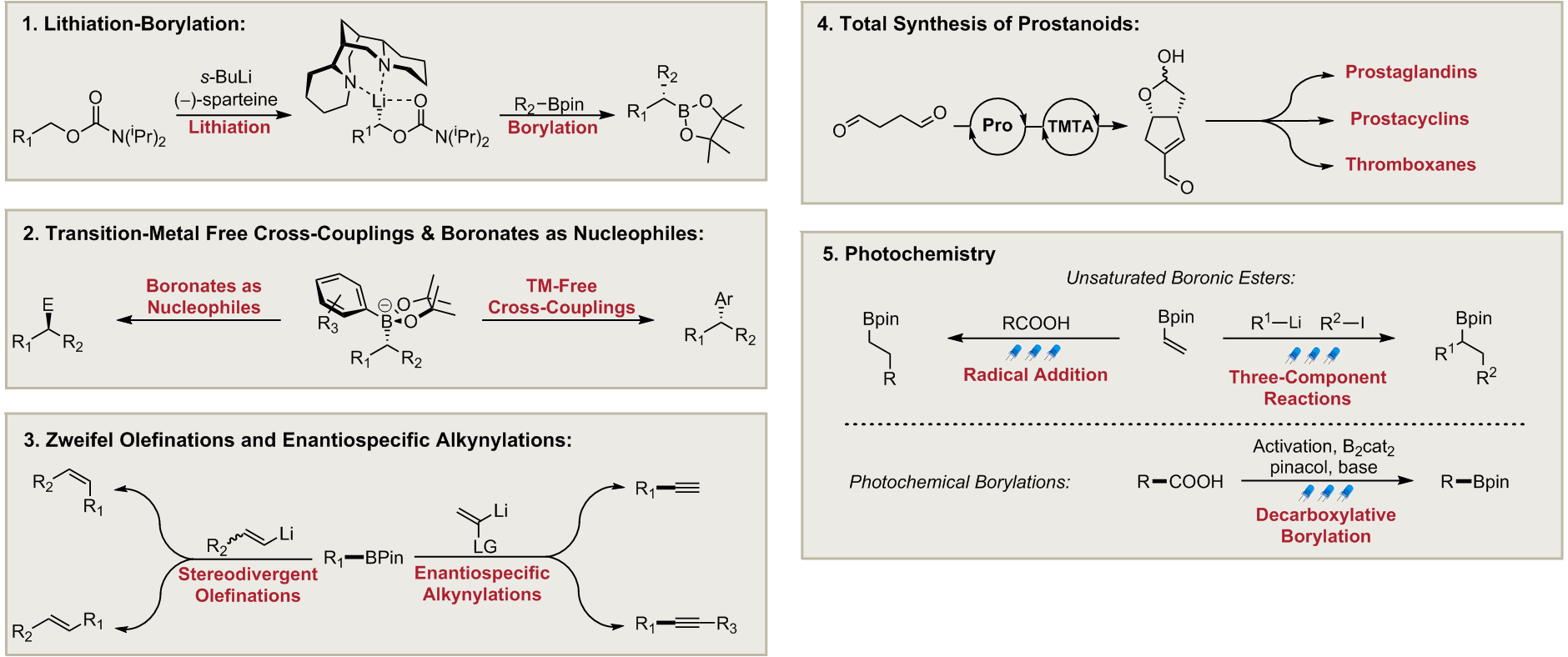
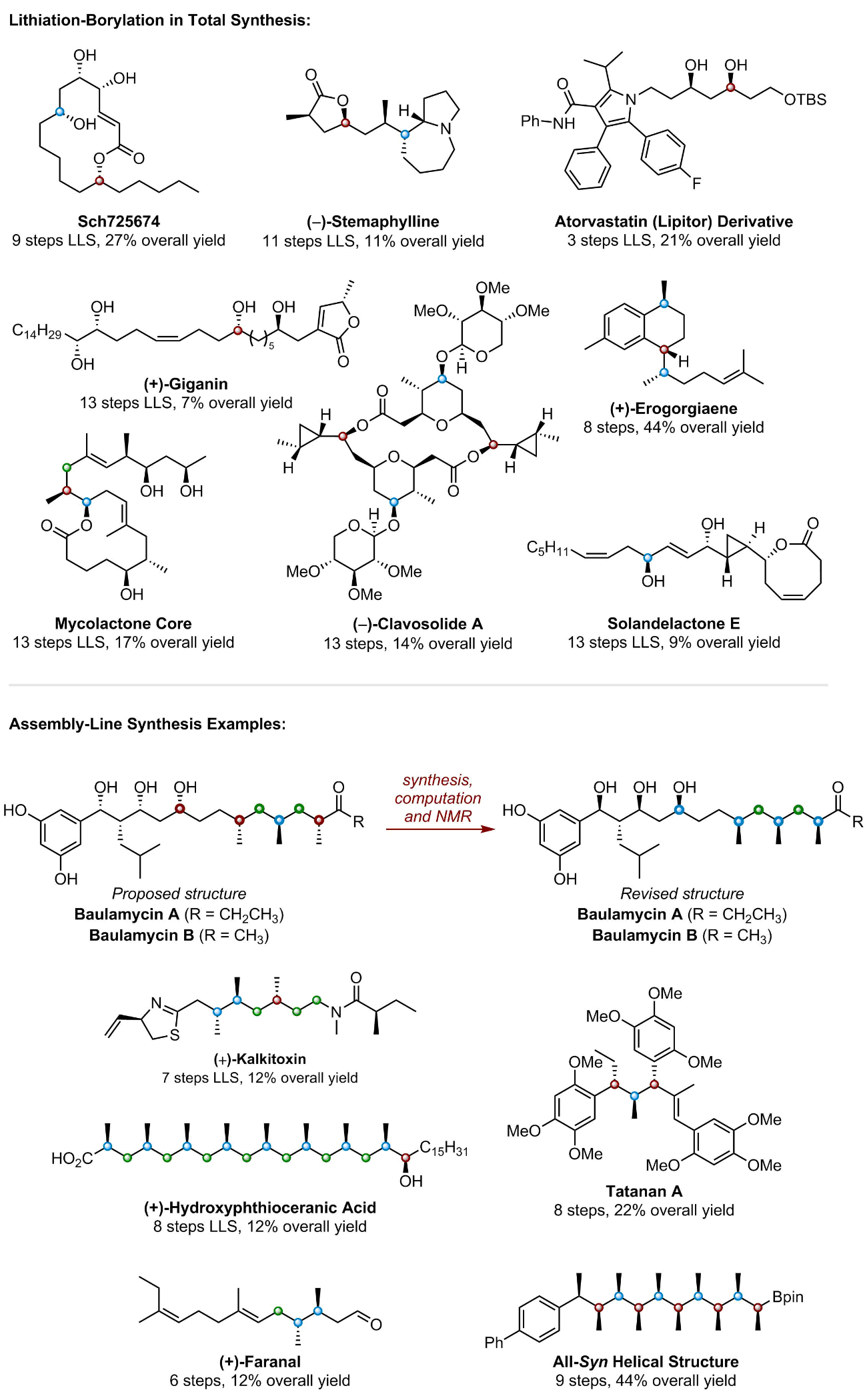
Lithiation–borylation is an invaluable carbon-carbon bond forming technique, which has been both pioneered and used extensively within our group to homologate organoboron species in high yields with excellent stereocontrol. This technique has allowed us to access to a wide-range of complex 3D structures that were previously inaccessible by traditional synthetic means [1-4].
The versatility of organoboron chemistry allows for the homologated organoboron species to be utilised in a number of ways, a prominent example of this is in Assembly-Line Synthesis. This is where up to 4 consecutive lithiation–borylation reactions can be carried out iteratively with exquisite 3D control to produce multiple contiguous stereogenic centres before a single aqueous work-up is required [5, 6].
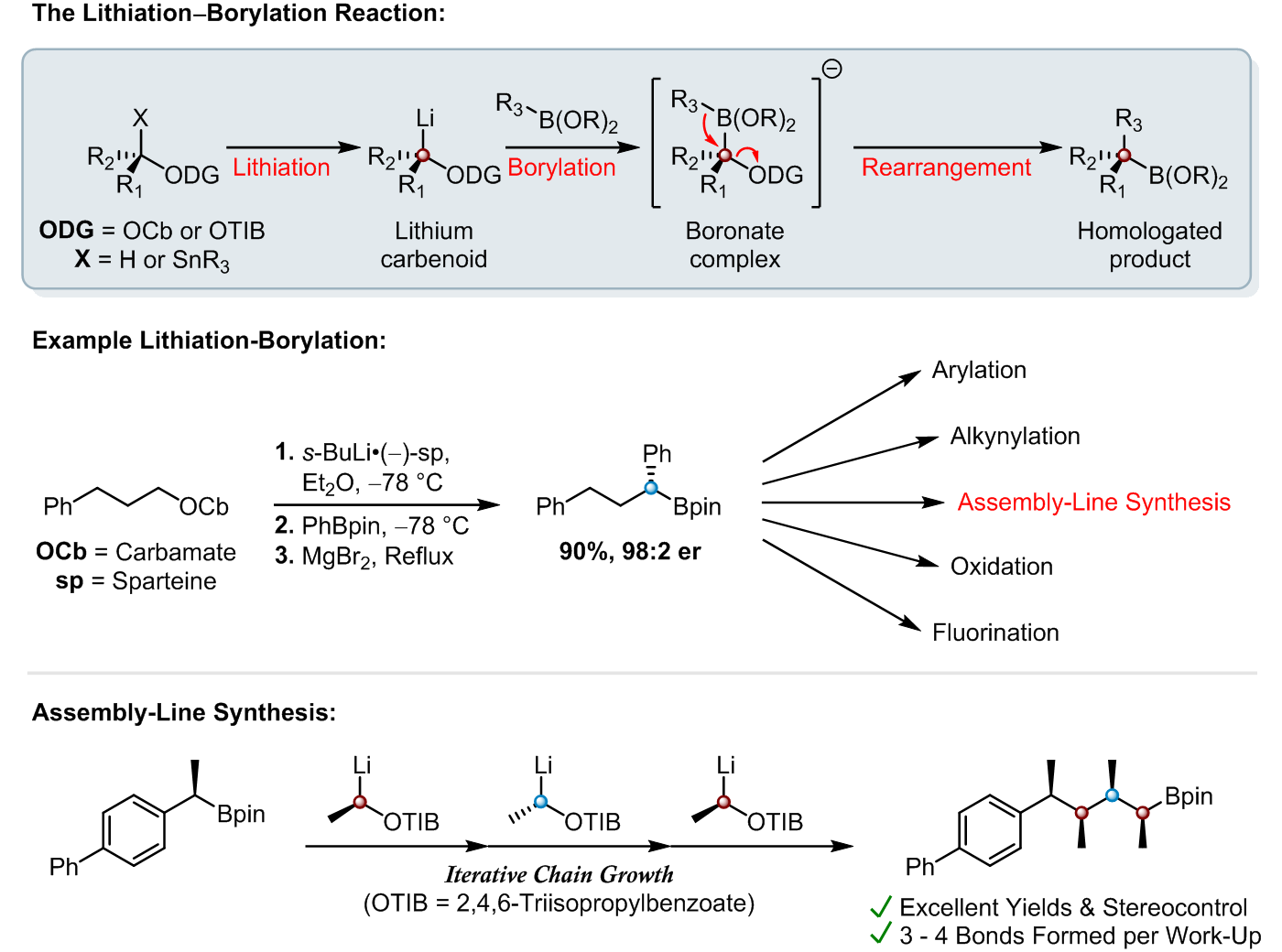
Assembly-Line Synthesis mimics the way that complex natural products are biosynthesised in nature by essentially “growing” molecules with total control over their 3D shape [5, 6]. This has been demonstrated well within the context of total synthesis, where our group has quickly produced a number of synthetically challenging 3D structures [6-8]. Recently assembly-line synthesis was used to efficiently prepare an encoded mixture of natural product diastereomers which were combined with predicted NMR parameters to identify and synthesize the correct structures of Baulamycins A and B [9].
References
[1] Leonori, D., Aggarwal, V. K., Acc. Chem. Res., 2014, 47, 3174–3183 doi
[2] Essafi, S., Tomasi, S., Aggarwal, V. K., Harvey, J. N., J. Org. Chem., 2014, 79, 12148 − 12158 doi
[3] Fawcett, A., Nitsch, D., Ali, M., Bateman, J. M., Myers, E. L., Aggarwal, V. K., Angew. Chem. Int. Ed., 2016, 55, 14663–14667 doi
[4] Varela, A., Garve, L. K. B., Leonori, D., Aggarwal, V. K., Angew. Chem. Int. Ed., 2017, 56, 2127–2131 doi
[5] Burns, M., Essafi, S., Bame, J. R., Bull, S. P., Webster, M. P., Balieu, S., Dale, J. W., Butts, C. P., Harvey, J. N., Aggarwal, V. K., Nature, 2014, 513, 183–188 doi
[6] Balieu, S., Hallett, G. E., Burns, M., Bootwicha, T., Studley, J., Aggarwal, V. K., J. Am. Chem. Soc., 2015, 137, 4398–4403 doi
[7] Noble, A., Roesner, S., Aggarwal, V. K., Angew. Chem. Int. Ed., 2016, 55, 15920–15924 doi
[8] Bootwicha, T., Feilner, J. M., Myers, E. L., Aggarwal, V. K., Nature Chem., 2017, 9, 896–902 doi
[9] Wu, J., Lorenzo, P., Zhong, S., Ali, M., Butts, C. P., Myers, E. L., Aggarwal, V. K., Nature, 2017, 547, 436–440 doi
Transition-Metal Free Cross-Couplings
Cross-coupling reactions account for the formation of over 60% of C-C bonds in the pharmaceutical industry. However, traditional cross-coupling reactions often rely heavily on the use of expensive or toxic transition-metal catalysts. As such, there is significant interest in developing more efficient transition-metal free methods.
Recently, our group has successfully developed new methodology towards transition-metal free cross-coupling reactions based upon our lithiation-borylation chemistry. The methodology involves the formation of a chiral boronate complex followed by an activation method that triggers an enantiospecific 1,2-metallate rearrangement. These methods allow for the reliable enantiospecific sp2-sp3 coupling of both secondary and tertiary boronic esters with a variety of aryl motifs [1-6].
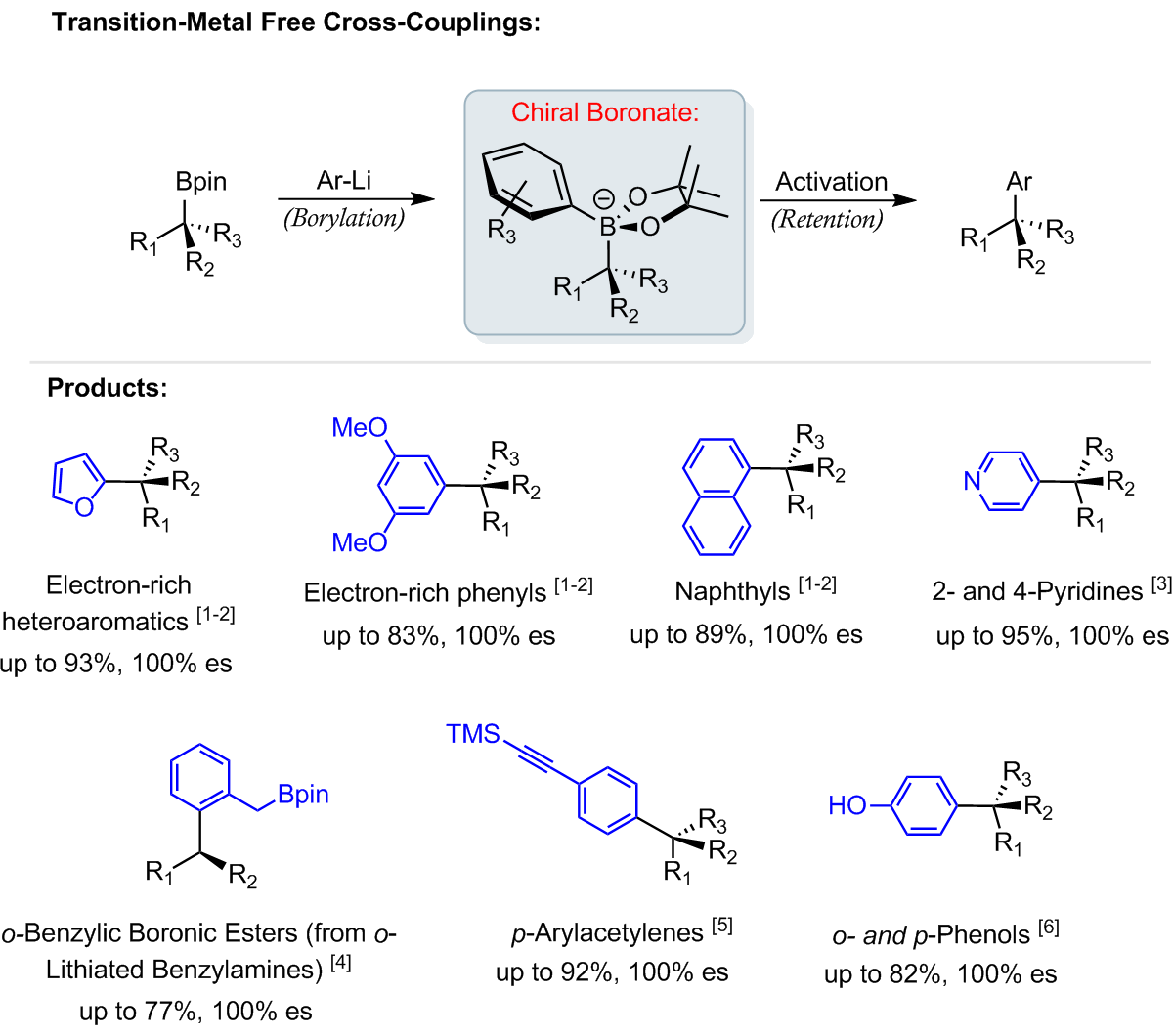
References
[1] Bonet, A., Odachowski, M., Leonori, D., Essafi, S., Aggarwal, V. K., Nat. Chem., 2014, 6, 584–589 doi
[2] Odachowski, M., Bonet, A., Essafi, S., Conti-Ramsden, P., Harvey, J. N., Leonori, D., Aggarwal, V. K., J. Am. Chem. Soc., 2016, 138, 9521–9532 doi
[3] Llaveria J., Leonori D., Aggarwal, V. K., J. Am. Chem. Soc., 2015, 137, 10958–10961 doi
[4] Aichhorn S, Bigler R., Myers E. L., Aggarwal V. K., J. Am. Chem. Soc., 2017, 139, 9519–9522 doi
[5] Ganesh V., Odachowski M., Aggarwal V. K., Angew. Chem. Int. Ed., 2017, 56, 9752–9756 doi
[6] Wilson C. M., Venkataraman G., Noble A., Aggarwal V. K., Angew. Chem. Int. Ed., 2017, 56, 16318–16322 doi
Boronates as Nucleophiles
Expanding upon our transition-metal free cross-coupling chemistry, we have developed an efficient method for the enantiospecific conversion of boronic esters into an array of synthetically useful functional groups [1, 2].
By trapping chiral boronic esters with readily-available electron-deficient organolithium reagents, we can form chiral boronates. These boronates act as organometallic-type nucleophiles, reacting with a wide-range of electrophiles and delivering synthetically useful products through the reliable net inversion of stereochemistry [1–3].
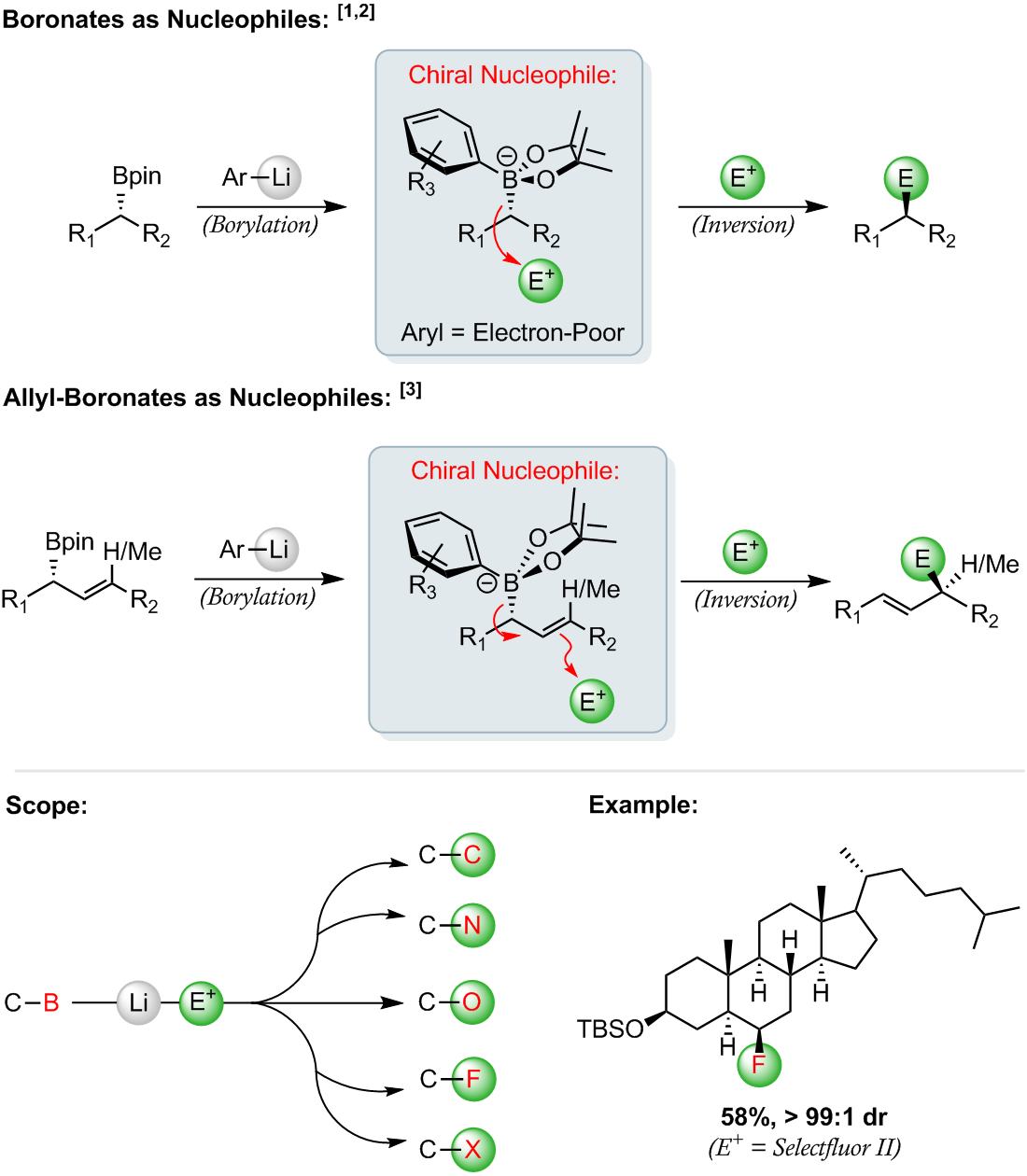
References:
[1] Larouche-Gauthier, R., Elford, T. G., Aggarwal, V. K., J. Am. Chem. Soc., 2011, 133, 16794–16797 doi
[2] Sandford, C., Rasappan, R., Aggarwal, V. K., J. Am. Chem. Soc., 2015, 137, 10100–10103 doi
[3] Garcia-Ruiz C., Chen J. L.-Y., Sandford C., Feeney K., Lorenzo P., Berionni G., Mayr H., Aggarwal V. K., J. Am. Chem. Soc., 2017, 139, 15324–15327 doi
[4] Sandford, C., Aggarwal, V. K., Chem. Commun., 2017, 53, 5481–5494 [Review] doi
Zweifel Olefinations and Enantiospecific Alkynylations
Our group has also successfully developed the transition-metal free stereodivergent coupling of vinyl halides with boronic esters, based upon the Zweifel olefination [1–3].
The process can be used to couple sp2 and chiral sp3 boronic esters with complete enantiospecificity, and most importantly, it allows for the highly stereoselective synthesis of either the E- or Z-alkene from a single isomer of vinyl coupling partner [1].
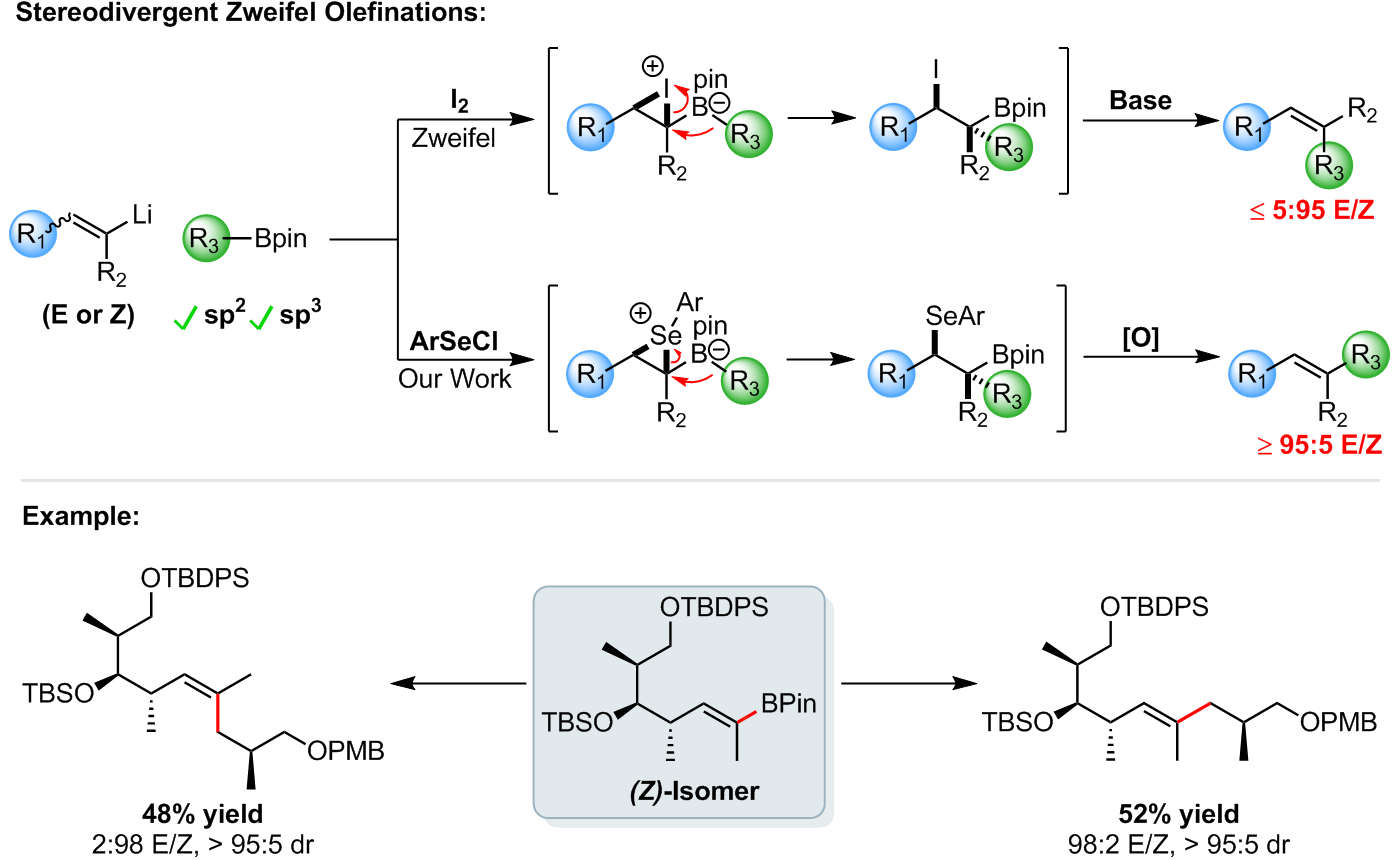
Expanding upon our Zweifel olefination methodology, we have also successfully developed a novel approach towards the enantiospecific deborylative alkynylation of enantioenriched secondary and tertiary boronic esters [4].
The process allows for the conversion of chiral boronic esters into terminal alkynes with high yields and excellent levels of enantiospecificity. Furthermore, internal and silyl-protected alkynes can also be produced with this process [4].
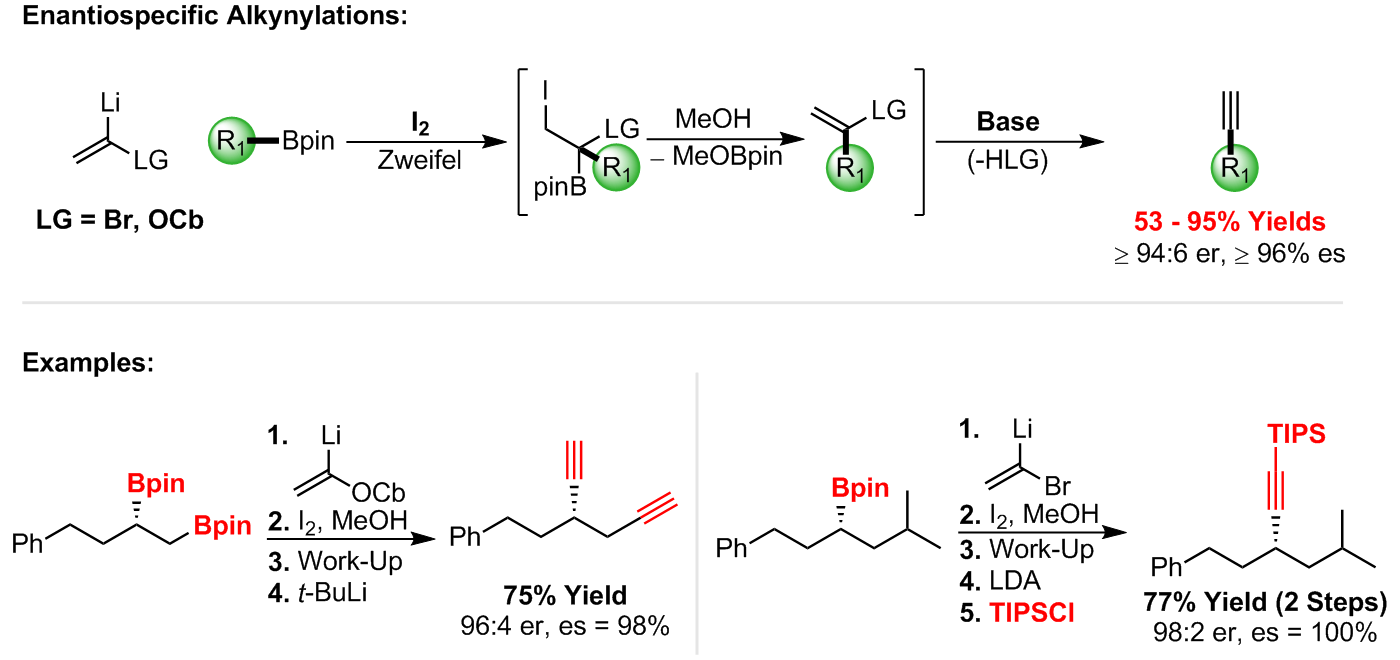
References
[1] Armstrong, R. J., García-Ruiz, C., Myers, E. L., Aggarwal, V. K., Angew. Chem. Int. Ed., 2017, 56, 786–790 doi
[2] Armstrong, R. J., Niwetmarin, W., Aggarwal, V. K., Org. Lett., 2017, 19, 2762–2765 doi
[3] Armstrong, R. J., Aggarwal, V. K., Synthesis, 2017, 49, 3323–3336 doi
[4] Wang, Y., Noble, A., Myers, E. L., Aggarwal, V. K., Angew. Chem. Int. Ed., 2016, 55, 4270–4274 doi
Total Synthesis of Prostanoids
Prostanoids are a family of biologically significant natural products derived from arachidonic acid, which are known to play crucial roles in inflammation, blood pressure control and platelet formation. Prostanoid total synthesis has been an active area of research since the 1970s and has led to the development of a number of impressive, yet lengthy, routes.
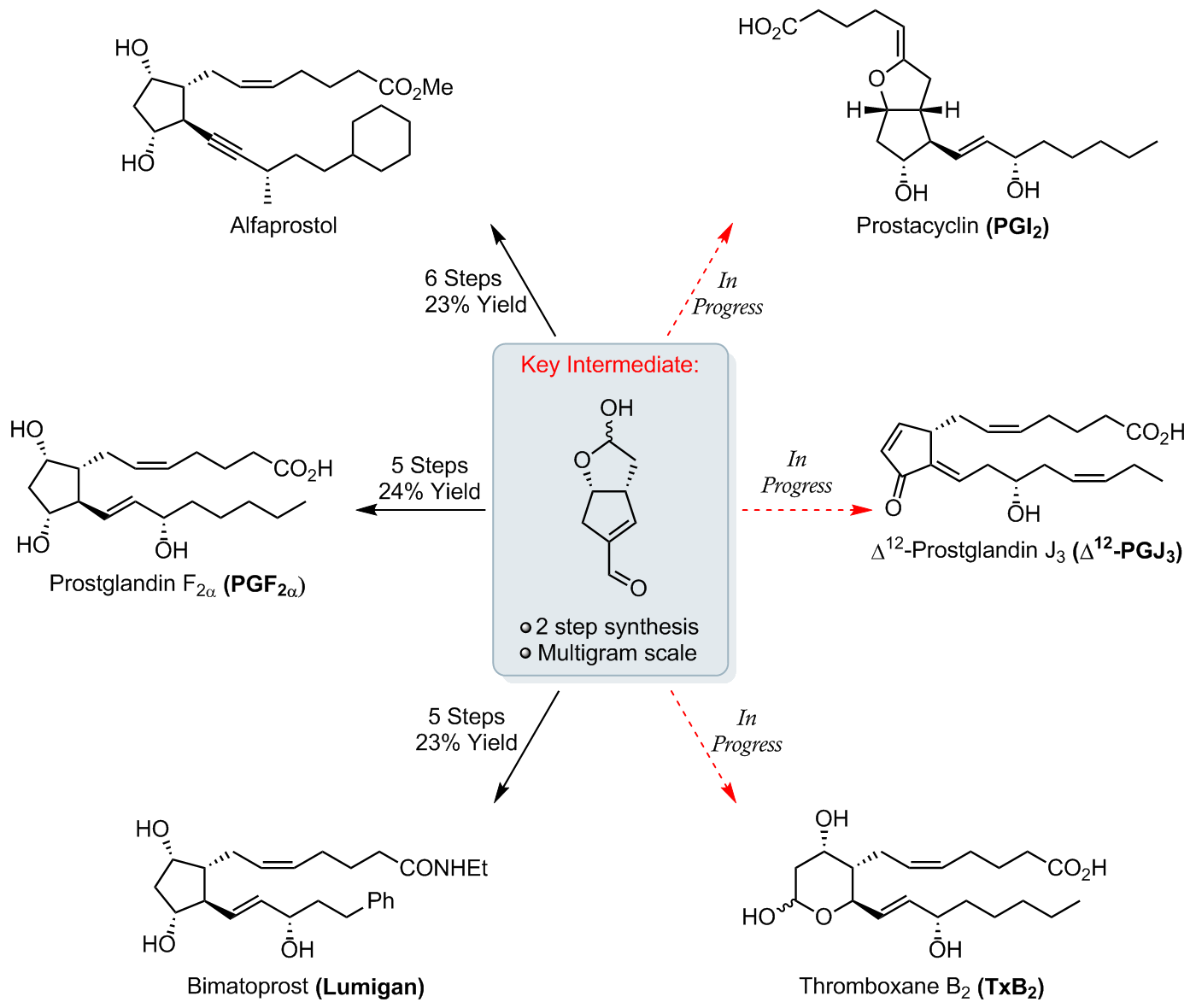
Our uniform strategy towards the total synthesis of various prostanoids and their derivatives exploits a key enal intermediate, which is prepared via a stereocontrolled organocatalytic dimerisation of succinaldehyde [1, 2].
This crystalline intermediate can be readily produced on gram-scale and serves as an advanced intermediate for the quick and efficient synthesis of a number of complex prostanoids, including PGF2α, a medicinally important natural product that was synthesised by our group on gram-scale in only 7 steps [1–3].
References
[1] Coulthard, G., Erb, W., Aggarwal, V. K., Nature, 2012, 489, 278–281 doi
[2] Prévost, S., Thai, K., Schützenmeister, N., Coulthard, G., Erb, W., Aggarwal, V. K., Org. Lett., 2015, 17, 504–507 doi
[3] H. Baars, M. J. Classen, V. K. Aggarwal, Org. Lett., 2017, 19, 6008–6011 doi
Photochemistry
Photochemical transformations have recently emerged as a powerful tool for the development of novel methodologies in organic synthesis and the construction of complex scaffolds.
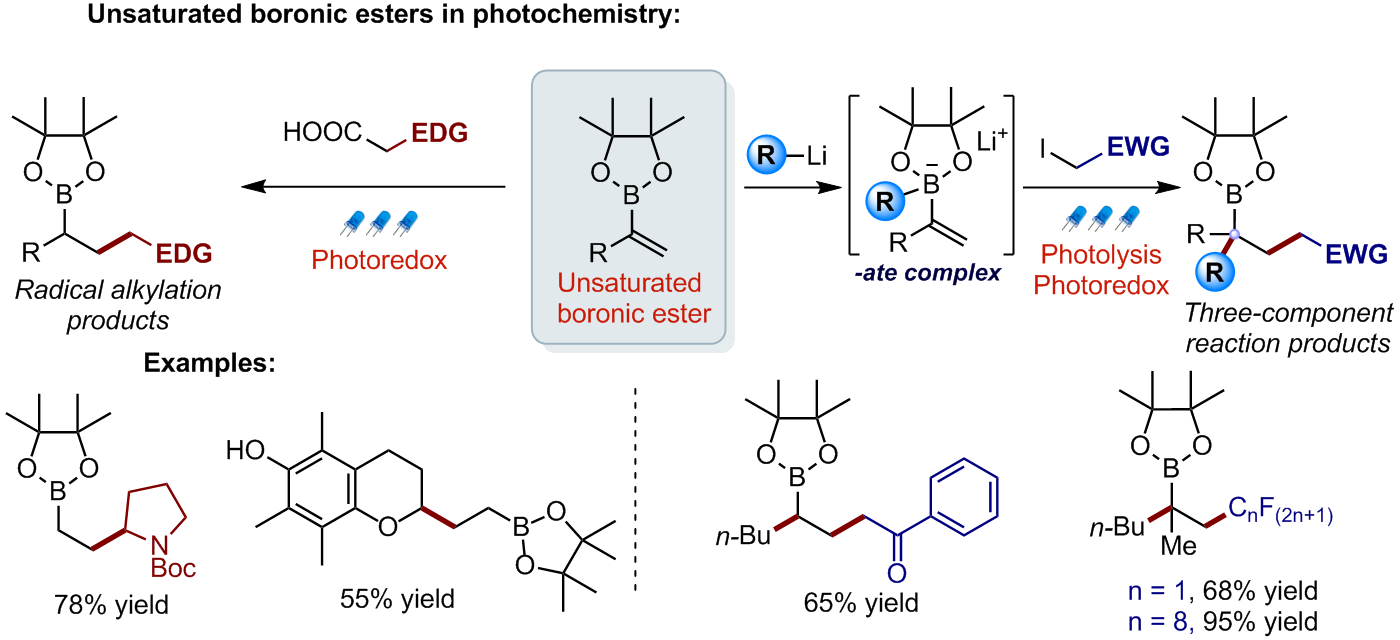
Exploiting the great potential of photoredox catalysis, we have developed novel radical transformations of unsaturated boronic esters. Vinyl boronic esters react with alkyl radicals generated from carboxylic acids, affording radical alkylation products in high yield [1]. In addition, boronate complexes generated from analogous vinyl boronic esters could be efficiently reacted with electrophilic radicals generated by visible light irradiation of an alkyl iodide, affording three component reaction products [2].
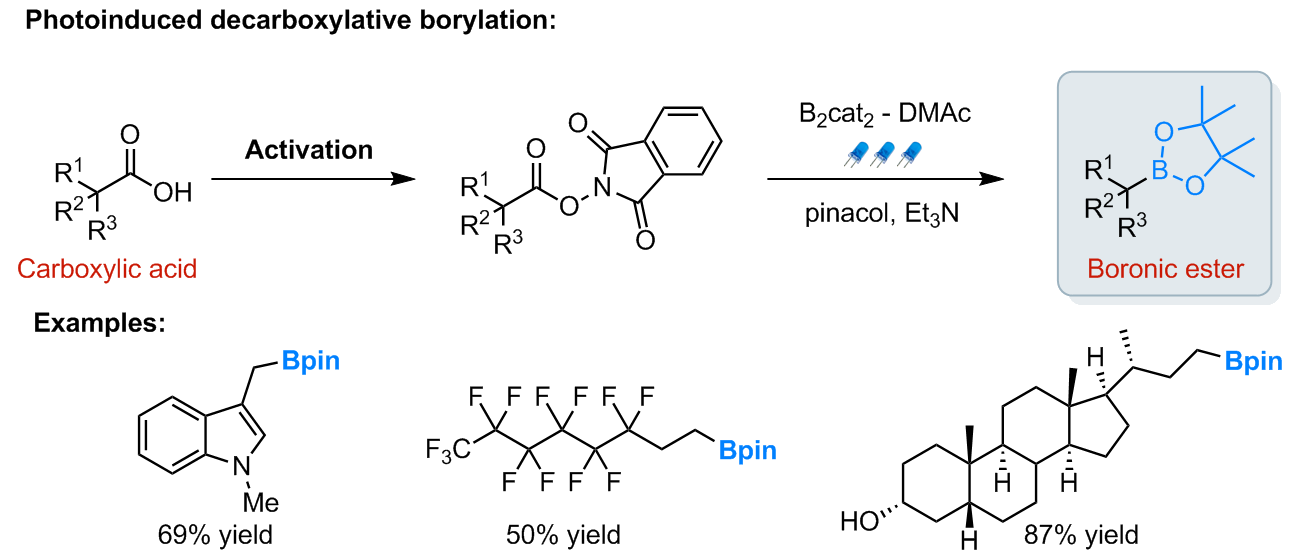
In view of the great opportunities offered by organoboron chemistry, new mild synthetic methods for the introduction of boron-containing motifs in organic molecules are highly desirable. We have recently reported a photoinduced-radical borylation of redox active esters generated from readily available carboxylic acids. The methodology gives access to a wide array of boronic ester products in good yields [3].
References:
[1] Noble, A.; Mega, R. S.; Pflästerer, D.; Myers, E. L.; Aggarwal, V. K. Angew. Chem. Int. Ed., 2018, 57, 2155–2159 doi
[2] Silvi, M.; Sandford, C.; Aggarwal, V. K. J. Am. Chem. Soc. 2017, 139, 5736–5739 doi
[3] Fawcett, A.; Pradeilles, J.; Wang, Y.; Mutsuga, T.; Myers, E. L.; Aggarwal, V. K. Science 2017, 357, 283–286 Online PDF
(2018 revision: Sheenagh Aiken, Charlotte Gregson, Mattia Silvi, Oleksandr Zhurakovskyi. Original version: Steven Bennett, Oleksandr Zhurakovskyi.)