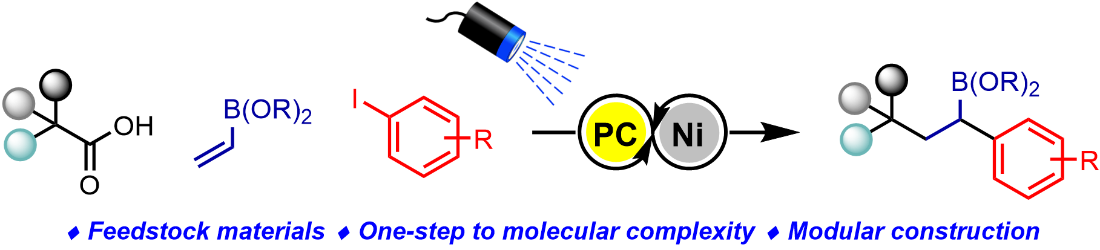
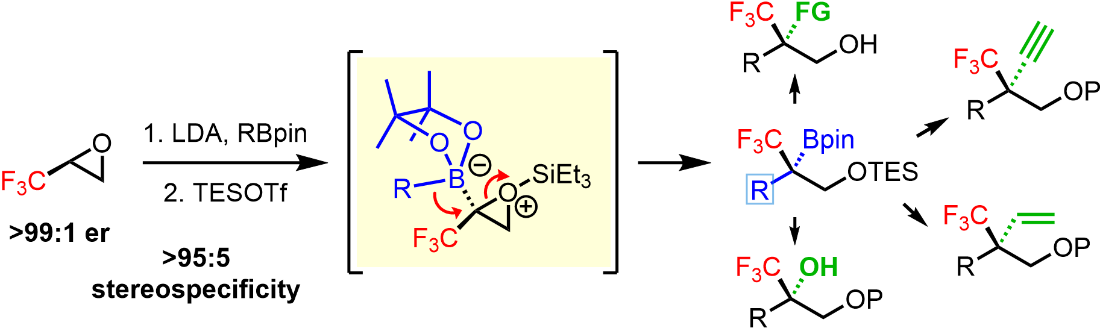
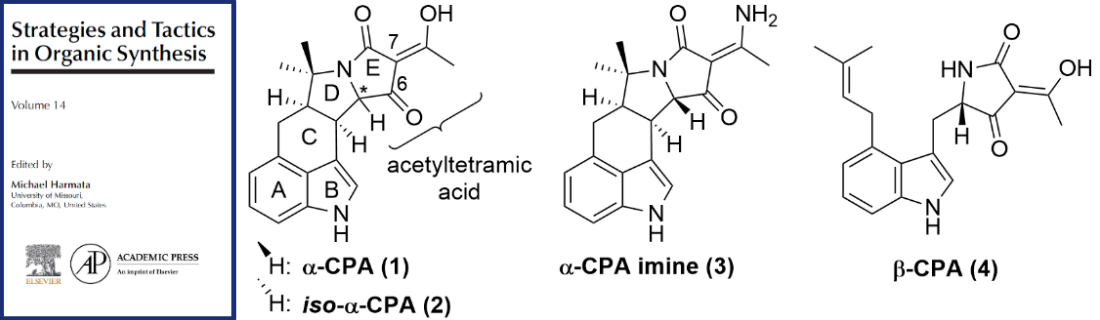
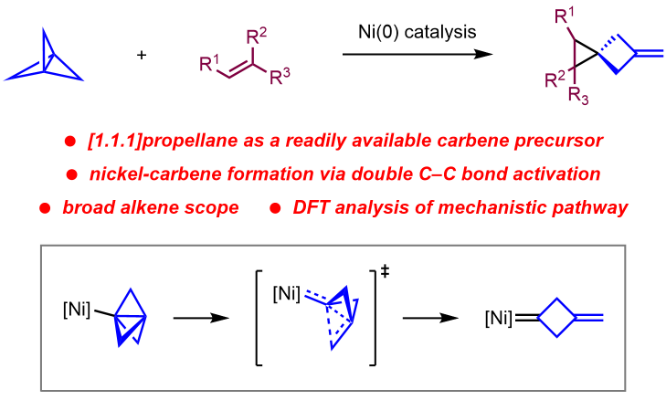
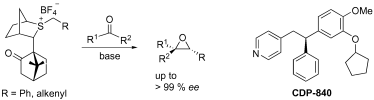Publication List
2024
2023
2022
2021
2020
2019
2018
2017
2016
2015
2014
2013
2012
2011
2010
2009
2008
2007
2006
2005
Pre-2005
2025
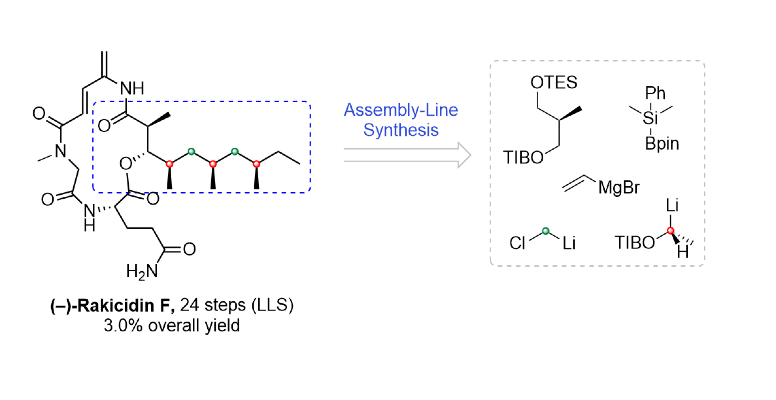
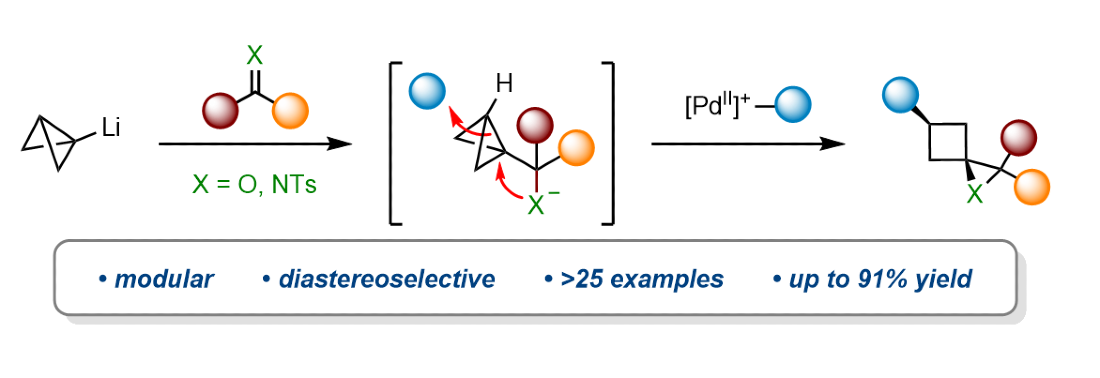
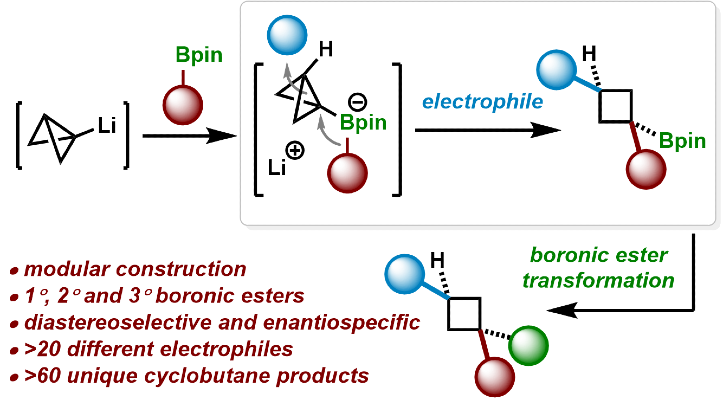
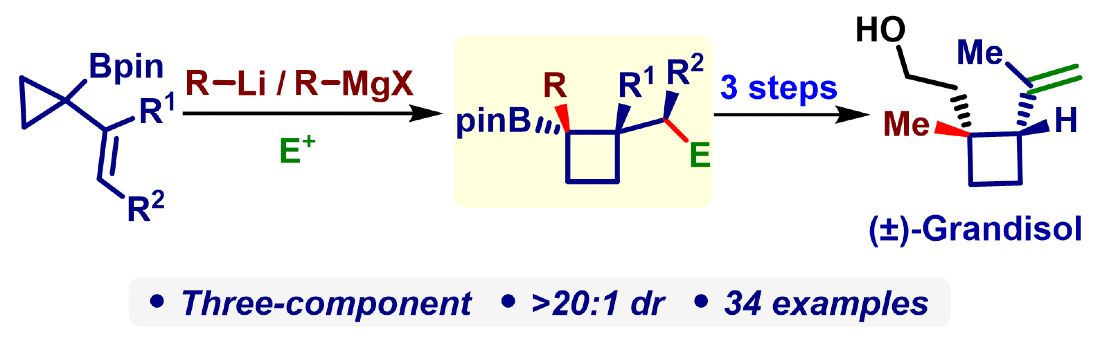
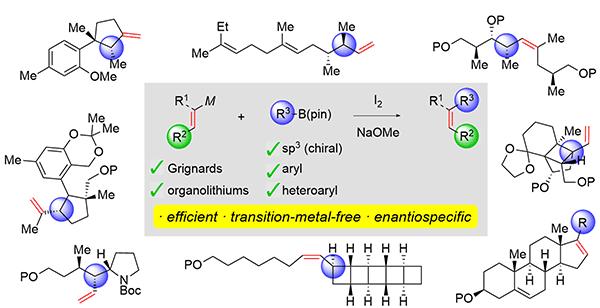
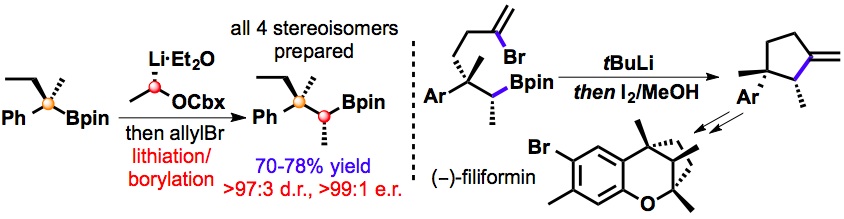
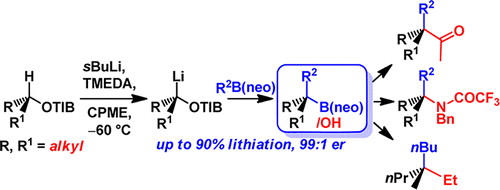
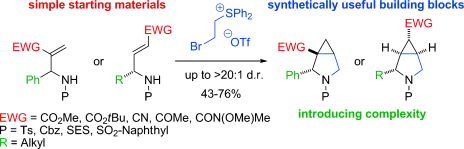
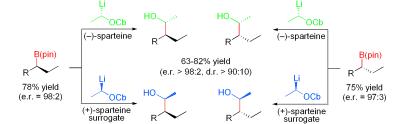
411. Intramolecular Lithiation-Borylation for the Stereoselective Synthesis of Cyclopentyl and Cyclobutyl Bis-Boronic Esters.
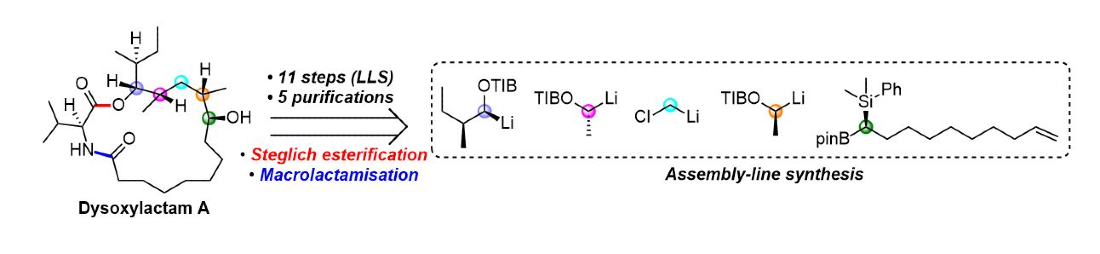
410. Total Synthesis and Structure Determination of Mycapolyol E Using Iterative Homologation of Boronic Esters.
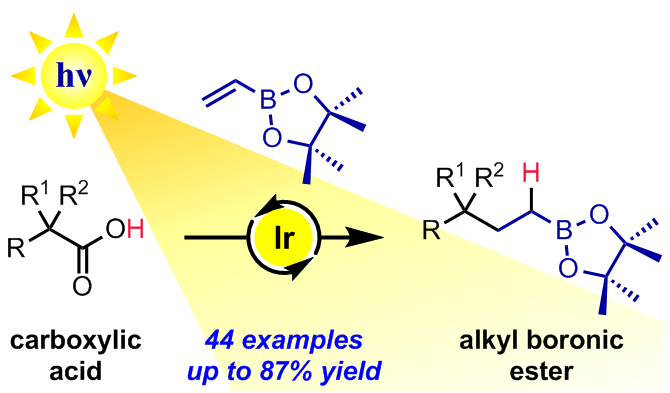
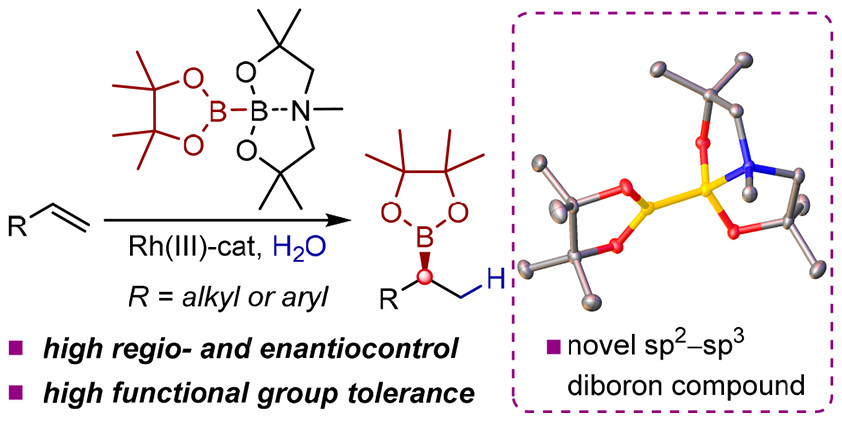
408. Persistent Boryl Radicals as Highly Reducing Photoredox Catalysts for Debrominative Borylations.
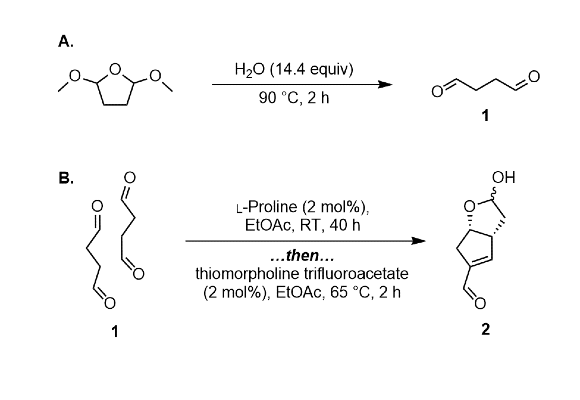
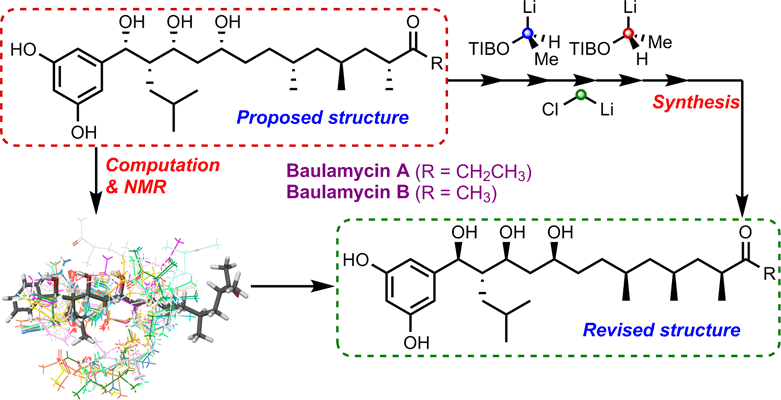
406. The combination of synthesis and ultra-high-resolution NMR spectroscopy reveals the correct structure of caylobolide A
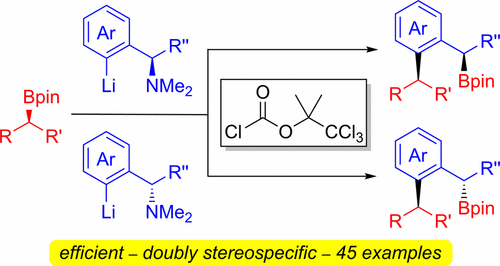
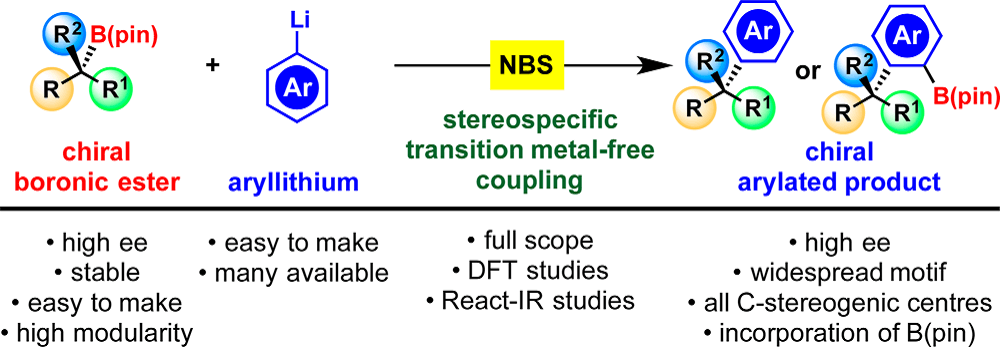
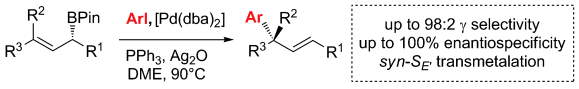
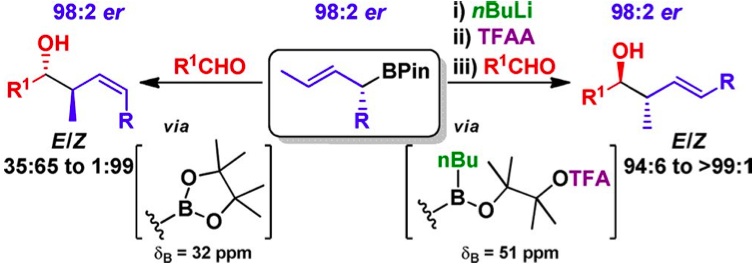
405. Iridium-Catalyzed Stereocontrolled C(sp3)–C(sp3) Cross-Coupling of Boronic Esters and Allylic Carbonates Enabled by Boron-to-Zinc Transmetalation
2024
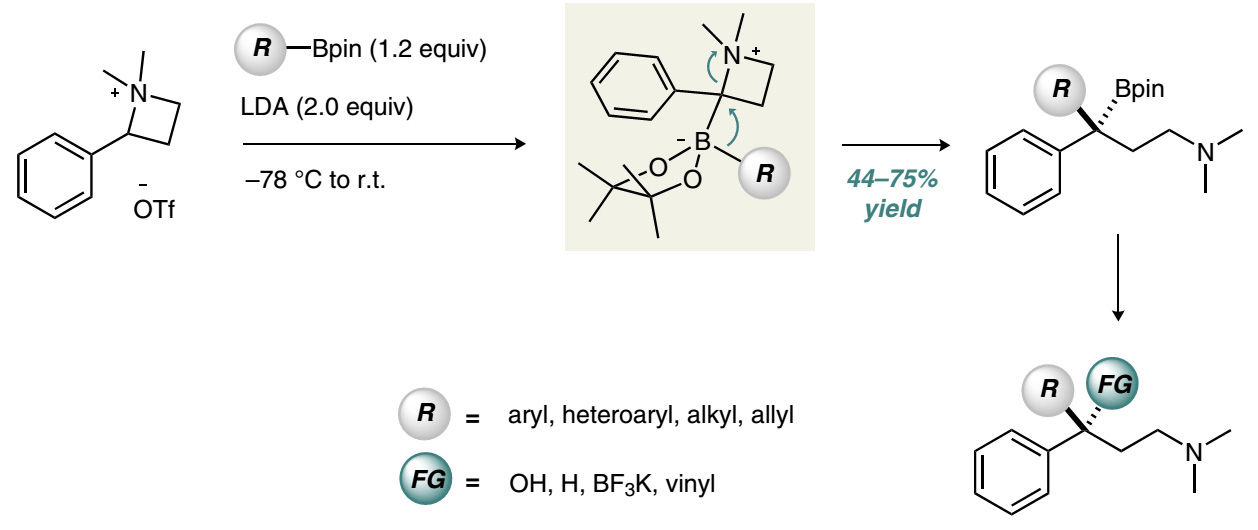
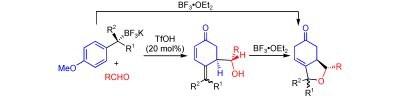
404. Merging Organocatalysis with 1,2-Boronate Rearrangement: A Lewis Base-Catalyzed Asymmetric Multicomponent Reaction
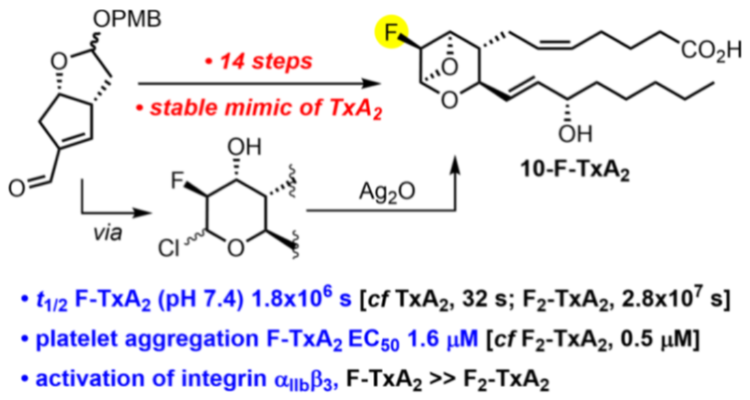
403. Platinum-catalyzed regio- and enantioselective diboration ofunactivated alkenes with (pin)B−B(dan)
402. Difluorinated A2 reveals crosstalk between platelet activatory and inhibitory pathways by targeting both the TP and IP receptor
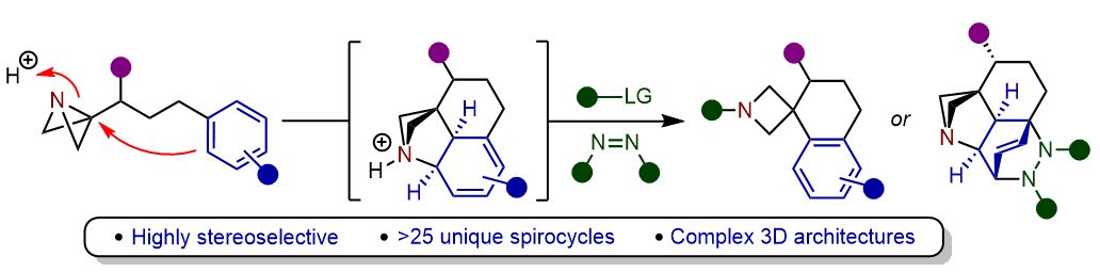
400. Synthesis of 1-azabicyclo[2.1.1]hexanes via formal singleelectron reduction of azabicyclo[1.1.0]butanes underphotochemical conditions
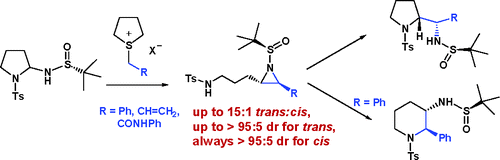
399. Simultaneous Stereoinvertive and Stereoselective C(sp3)–C(sp3) Cross-Coupling of Boronic Esters and Allylic Carbonates
2023
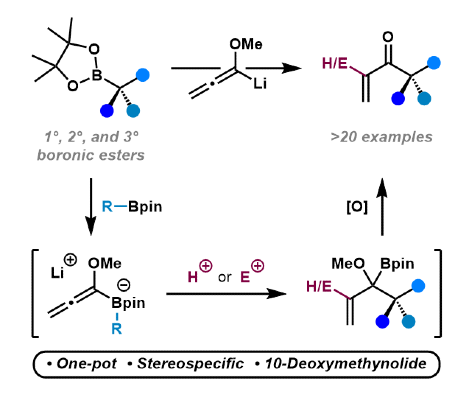
398. Stereospecific Conversion of Boronic Esters into Enones using Methoxyallene: Application in the Total Synthesis of 10-Deoxymethynolide
397. Convergent Deboronative and Decarboxylative Phosphonylation Enabled by the Phosphite Radical Trap “BecaP”
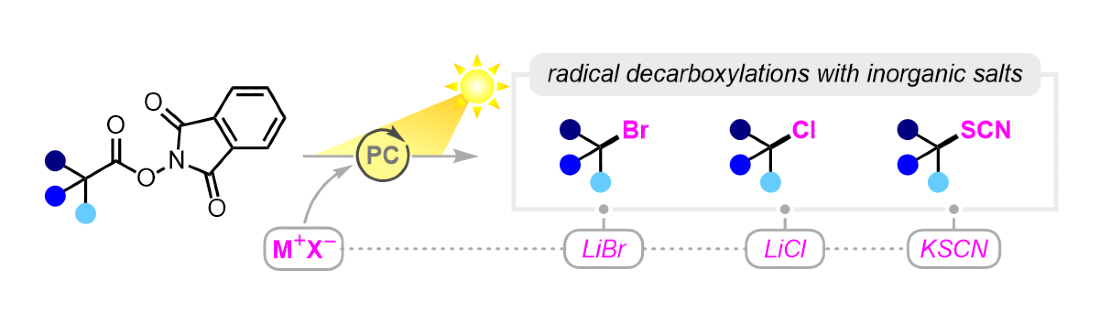
396. Photoredox-Catalyzed Decarboxylative Bromination, Chlorination and Thiocyanation Using Inorganic Salts
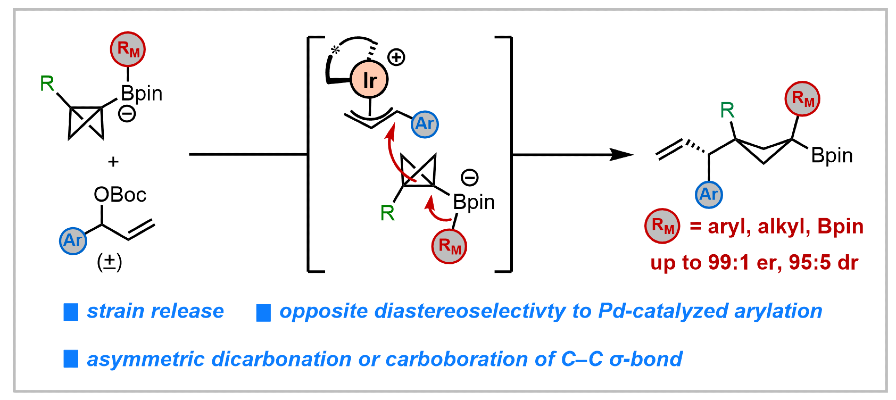
395. Iridium-Catalyzed Asymmetric Difunctionalization of C–C σ-Bonds Enabled by Ring-Strained Boronate Complexes
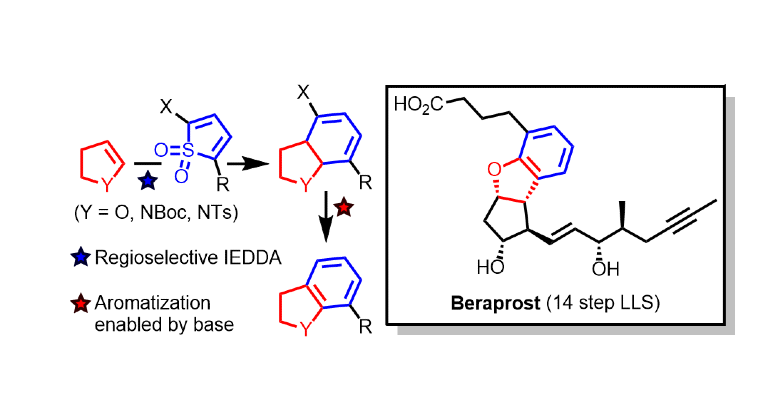
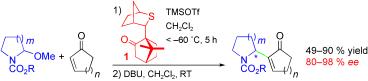
393. De Novo Synthesis of Dihydrobenzofurans and Indolines and Its Application to a Modular, Asymmetric Synthesis of Beraprost
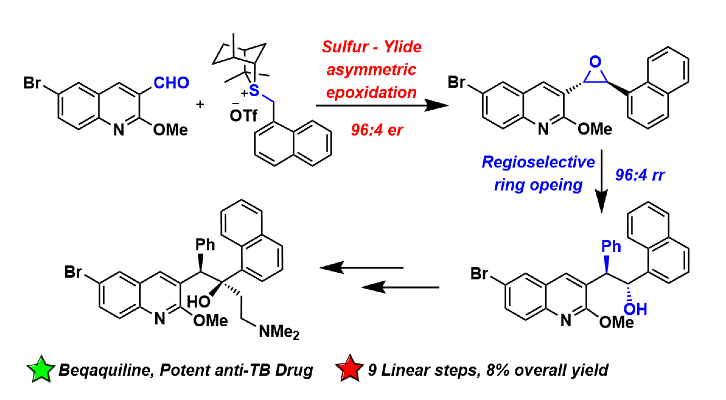
392. Application of Enantioselective Sulfur Ylide Epoxidation to a Short Asymmetric Synthesis of Bedaquiline, a Potent Anti-Tuberculosis Drug
2022
387. Strain-Release Driven Epoxidation and Aziridination of Bicyclo[1.1.0]butanes via Palladium Catalyzed σ-Bond Nucleopalladation
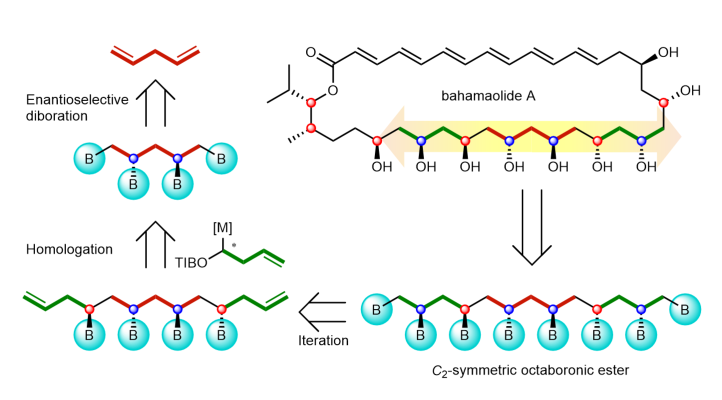
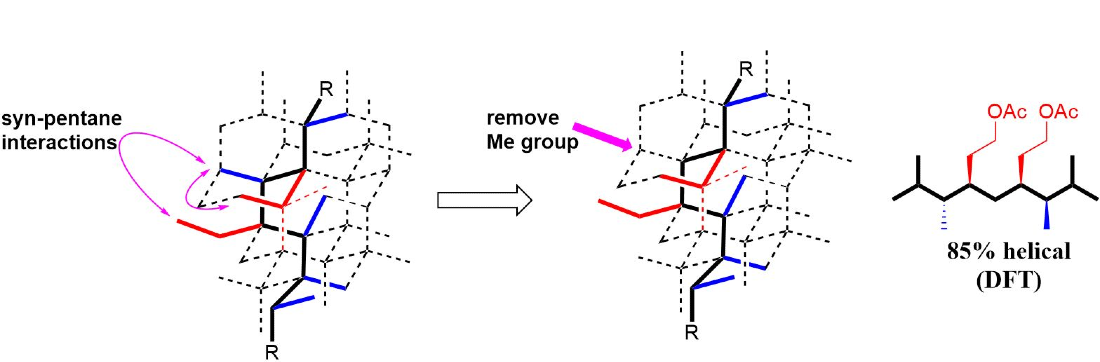
386. Iterative synthesis of 1,3-polyboronic esters with high stereocontrol and application to the synthesis of bahamaolide A
383. Dual-Gradient Unified Chromatography: A New Paradigm for Versatility in Simultaneous Multicomponent Analysis
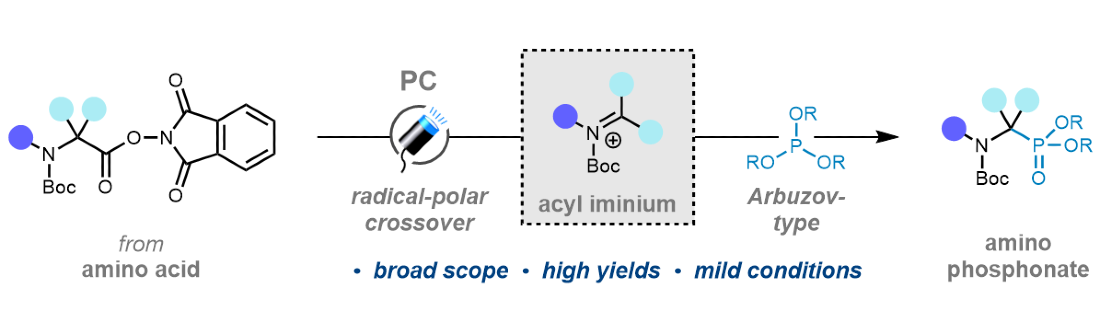
382. Facile Conversion of α-Amino Acids to α-Amino Phosphonates by Decarboxylative Phosphorylation using Visible-Light Photocatalysis
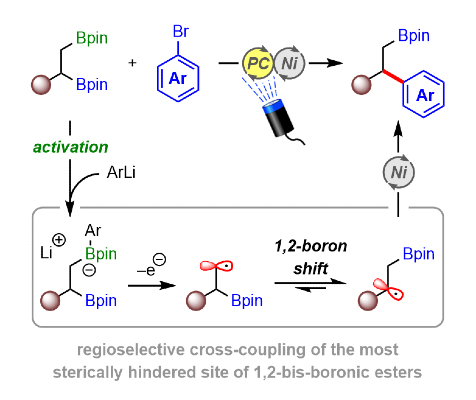
381. Dual Nickel/Photoredox-Catalyzed Site-Selective Cross-Coupling of 1,2-Bis-Boronic Esters Enabled by 1,2-Boron Shifts
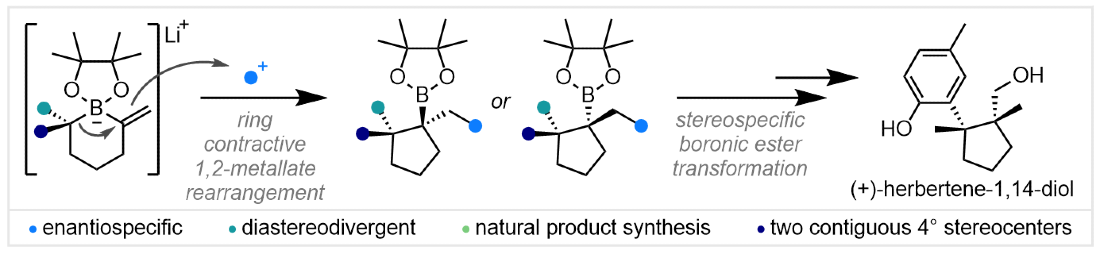
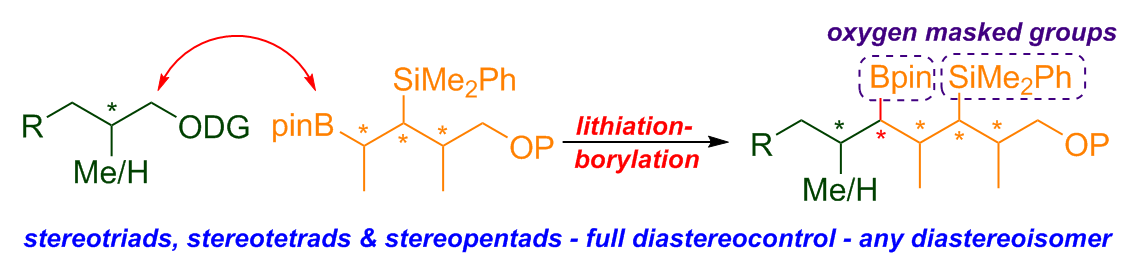
379. Diastereodivergent Synthesis of Cyclopentyl Boronic Esters Bearing Contiguous Fully Substituted Stereocenters
378. Stereocontrolled Total Synthesis of Bastimolide B Using Iterative Homologation of Boronic Esters
377. Selective Coupling of 1,2-Bis-Boronic Esters at the more Substituted Site through Visibile-Light Activation of Electron Donor-Acceptor Complexes
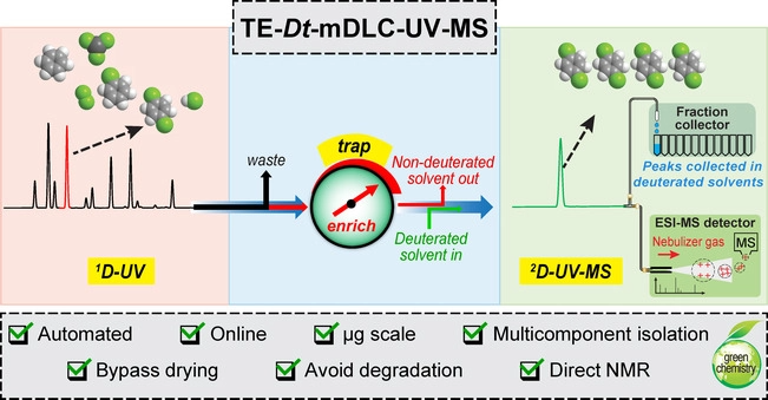
376. Trapping-Enrichment Multi-dimensional Liquid Chromatography with On-Line Deuterated Solvent Exchange for Streamlined Structure Elucidation at the Microgram Scale
2021
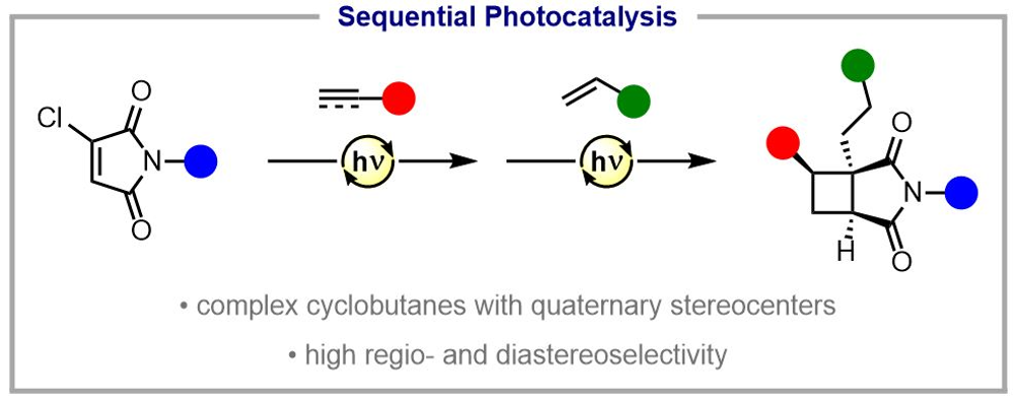
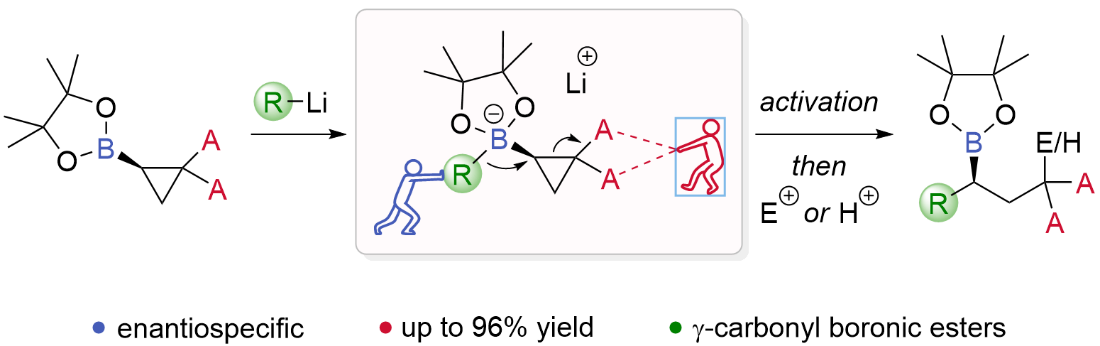
374. Sequential Photocatalytic Reactions for the Diastereoselective Synthesis of Cyclobutane Scaffolds
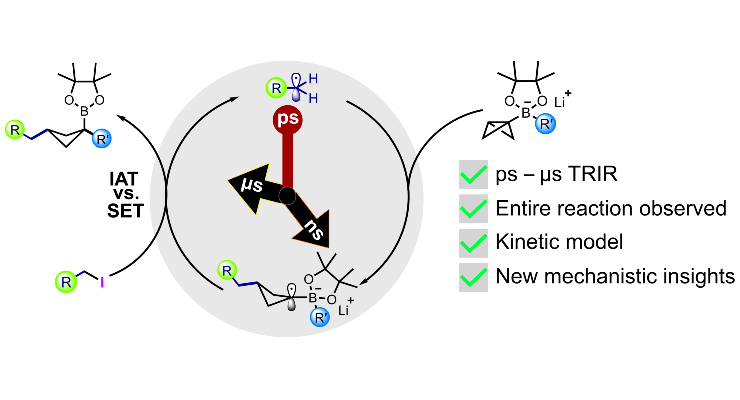
372. Direct Observation of Reactive Intermediates by Time-Resolved Spectroscopy Unravels the Mechanism of a Radical-Induced 1,2-Metalate Rearrangement
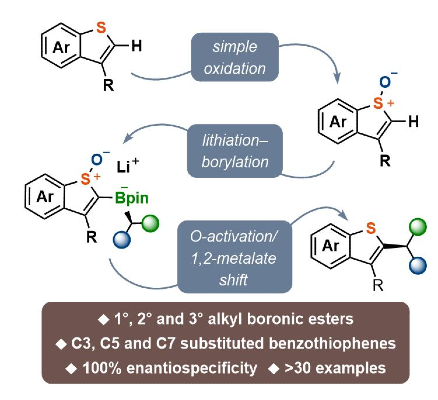
370. Chiral Benzothiophene Synthesis via Enantiospecific Coupling of Benzothiophene S-Oxides with Boronic Esters
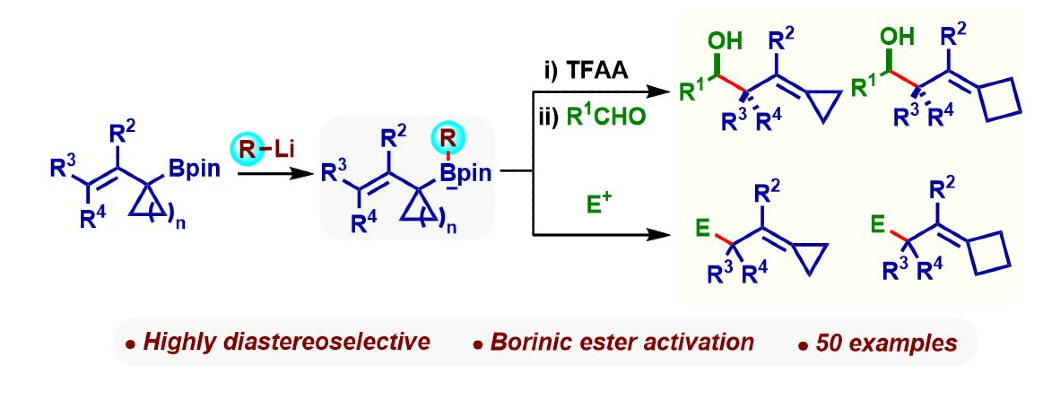
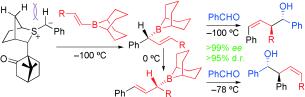
368. Highly Diastereoselective Strain-Increase Allylborations: Rapid Access to Alkylidenecyclopropanes and Alkylidenecyclobutanes
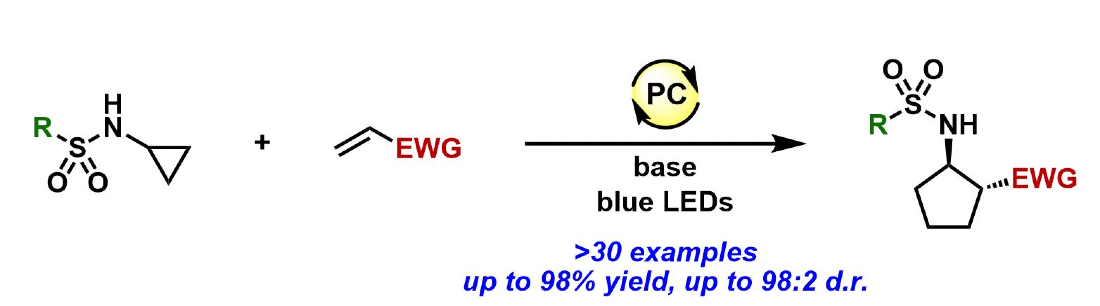
367. Diastereoselective Photoredox-Catalyzed [3 + 2] Cycloadditions of N-Sulfonyl Cyclopropylamines with Electron-Deficient Olefins
365. The Matteson Reaction
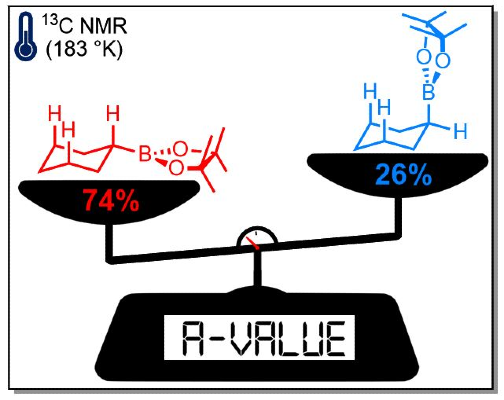
364. Studies on the lithiation, borylation, and 1,2-metalate rearrangement of O-cycloalkyl 2,4,6-triisopropylbenzoates
363. Divergent, Strain-Release Reactions of Azabicyclo[1.1.0]butyl Carbinols: Semipinacol or Spiroepoxy Azetidine Formation
362. Origin of stereocontrol in the Matteson reaction: Importance of attractive electrostatic interactions
2020
356. Difunctionalization of C–C σ Bonds Enabled by the Reaction of Bicyclo[1.1.0]butyl Boronate Complexes with Electrophiles: Reaction Development, Scope, and Stereochemical Origins
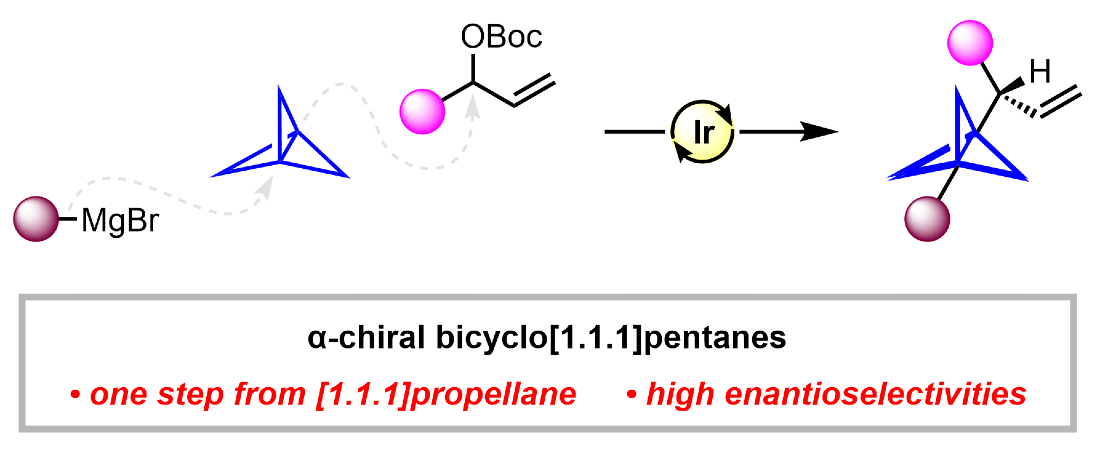
352. Iridium-Catalyzed Enantioselective Synthesis of Chiral Bicyclo[1.1.1]pentanes by 1,3-Difunctionalization of [1.1.1]Propellane
349. Ring-Expansion Induced 1,2-Metalate Rearrangements: Highly Diastereoselective Synthesis of Cyclobutyl Boronic Esters
346. Visible Light-Driven Strain-Increase Ring Contraction Allows the Synthesis of Cyclobutyl Boronic Esters
345. 1,3-Difunctionalizations of [1.1.1]Propellane via 1,2-Metallate Rearrangements of Boronate Complexes
344. Decarboxylative Conjunctive Cross-coupling of Vinyl Boronic Esters using Metallaphotoredox Catalysis
343. Ring-Opening Lithiation–Borylation of 2-Trifluoromethyl Oxirane: A Route to Versatile Tertiary Trifluoromethyl Boronic Esters
2019
339. Triphenylphosphine and sodium iodide: a new catalyst combination to rival precious metal complexes in visible light photoredox catalysis
337. 1,2-Boron Shifts of β-Boryl Radicals Generated from Bis-boronic Esters Using Photoredox Catalysis
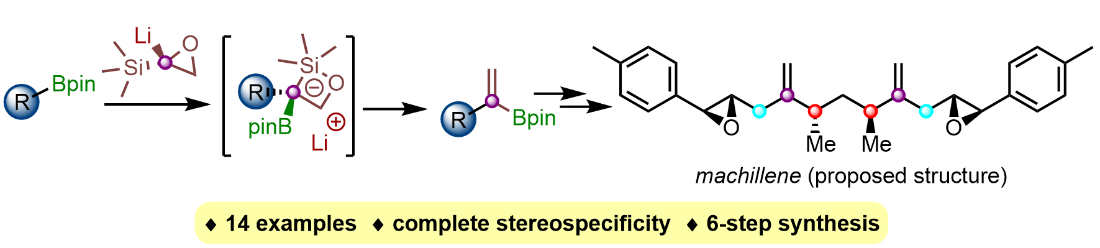
336. Vinylidene Homologation of Boronic Esters and its Application to the Synthesis of the Proposed Structure of Machillene
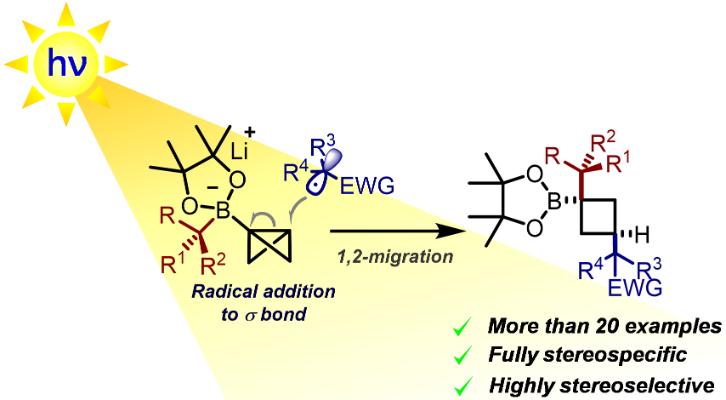
335. Radical Addition to Strained σ-Bonds Enables the Stereocontrolled Synthesis of Cyclobutyl Boronic Esters
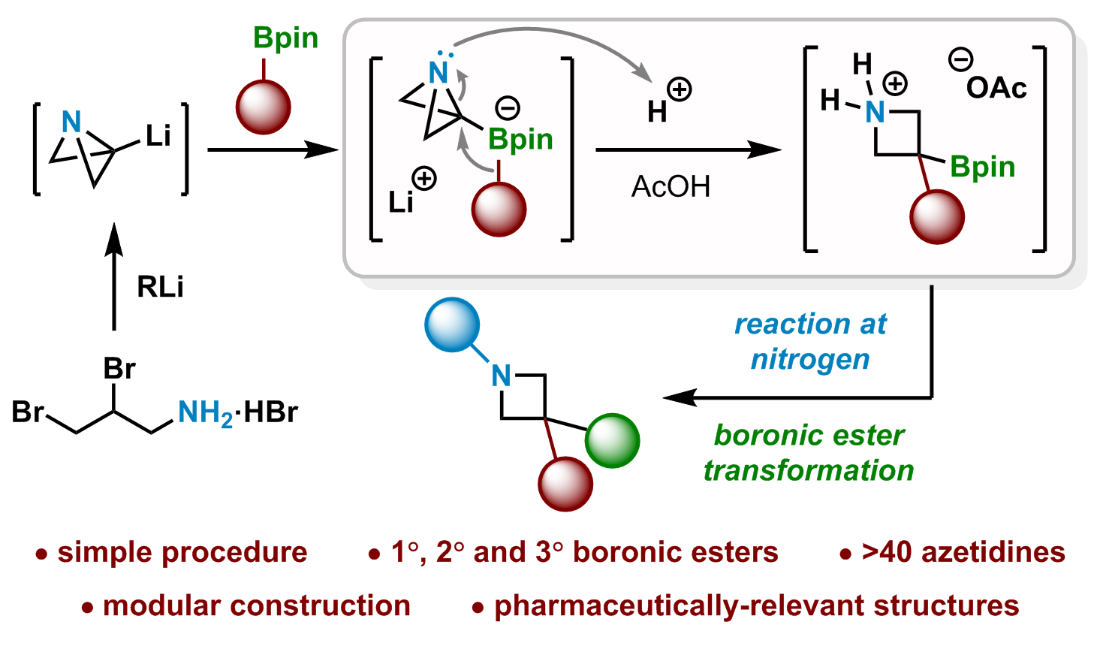
333. Strain-Release-Driven Homologation of Boronic Esters: Application to the Modular Synthesis of Azetidines
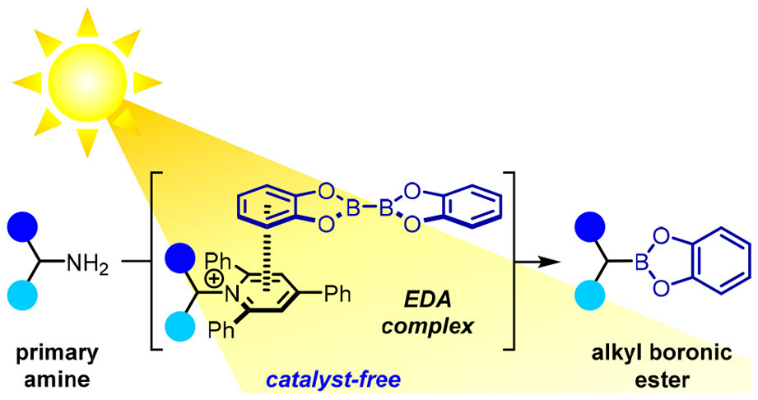
332. Catalyst-Free Deaminative Functionalizations of Primary Amines via Photoinduced Single-Electron Transfer
331. Photoredox‐Catalyzed Cyclobutane Synthesis via a Deboronative Radical Addition–Polar Cyclization Cascade
329. Enantiospecific Synthesis of ortho‐Substituted 1,1‐Diarylalkanes by a 1,2‐Metalate Rearrangement/anti‐SN2' Elimination/Rearomatizing Allylic Suzuki‐Miyaura Reaction Sequence
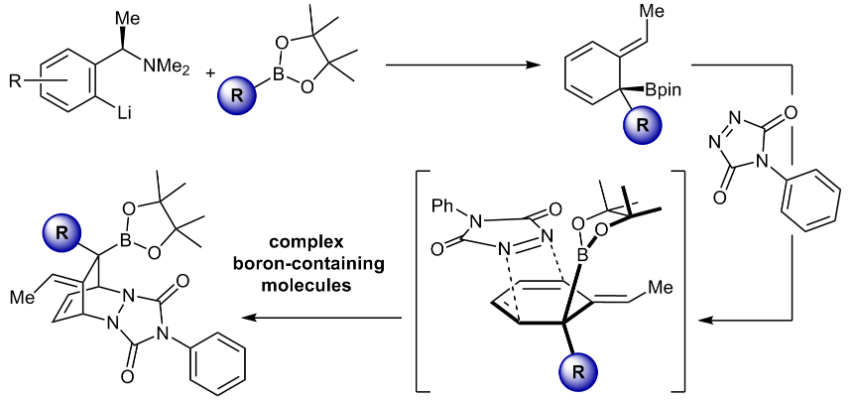
328. Complex Boron-Containing Molecules through a 1,2-Metalate Rearrangement/anti-SN2′ Elimination/Cycloaddition Reaction Sequence
2018
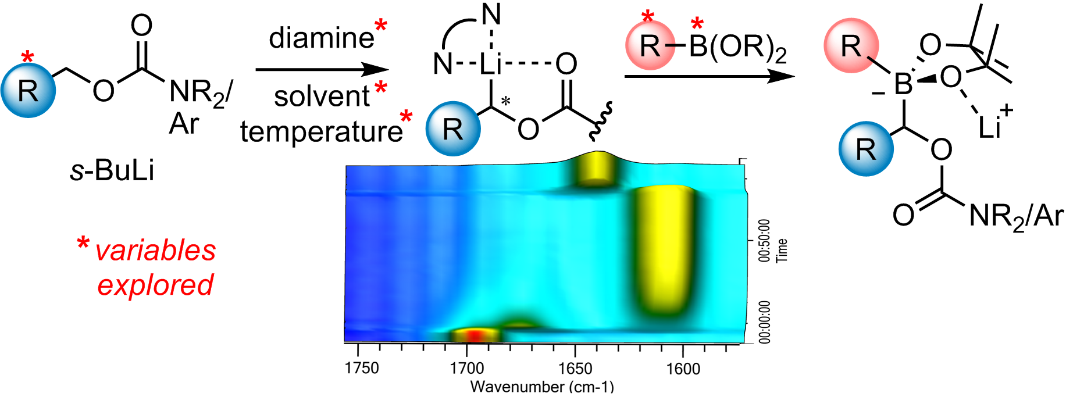
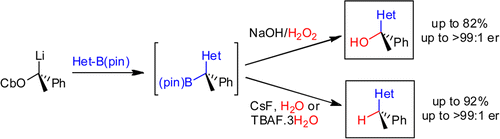
327. Investigation of the Deprotonative Generation and Borylation of Diamine-Ligated α-Lithiated Carbamates and Benzoates by in situ IR spectroscopy
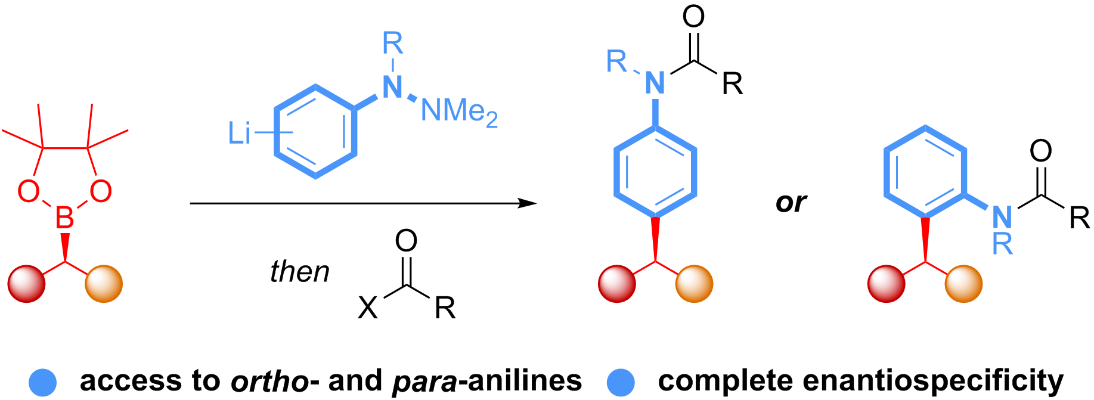
326. Chiral Aniline Synthesis via Stereospecific C(sp3)–C(sp2) Coupling of Boronic Esters with Aryl Hydrazines
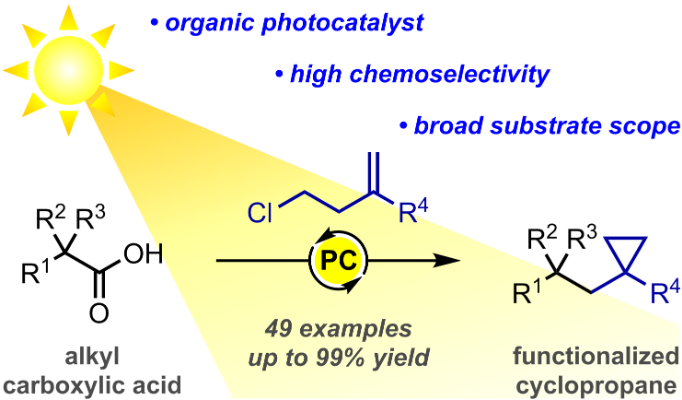
325. Synthesis of Functionalized Cyclopropanes from Carboxylic Acids via a Radical Addition‐Polar Cyclization Cascade
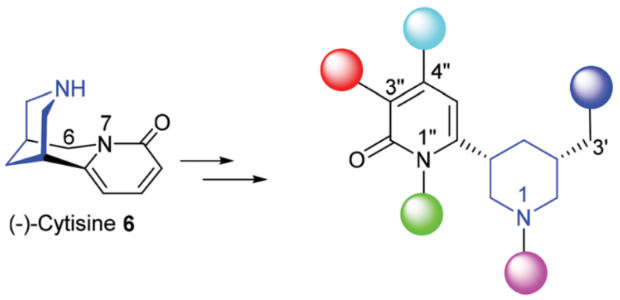
323. (–)-Cytisine: Access to a stereochemically defined and functionally flexible piperidine scaffold
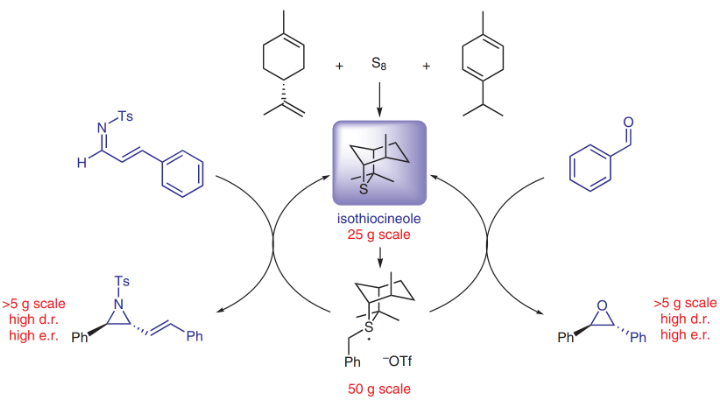
322. Synthesis of Isothiocineole and Application in Multigram-Scale Sulfur Ylide Mediated Asymmetric Epoxidation and Aziridination
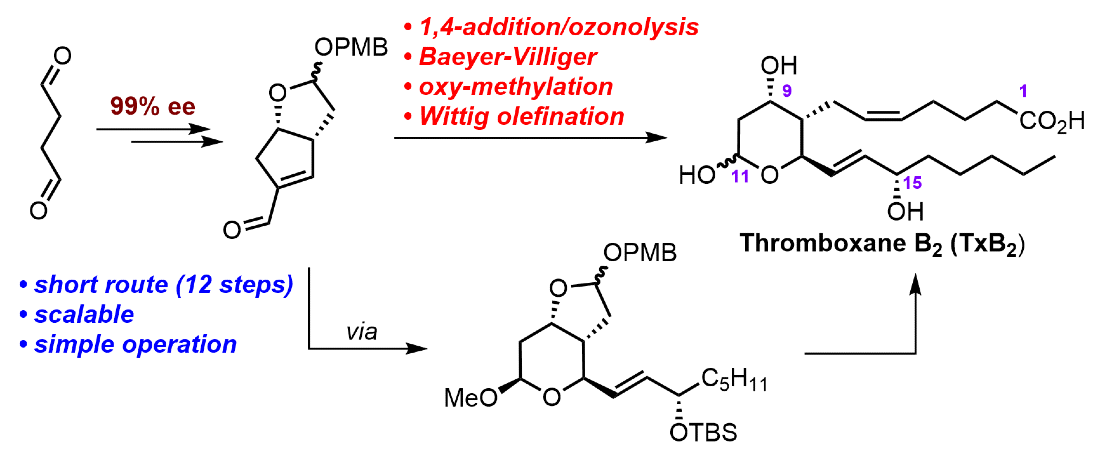
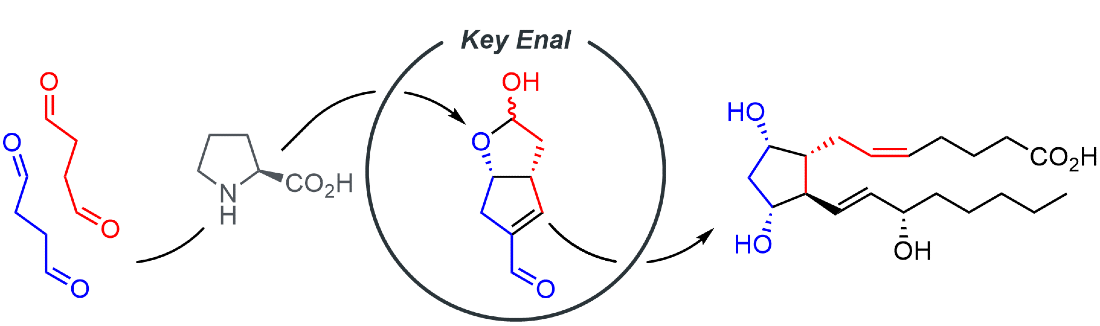
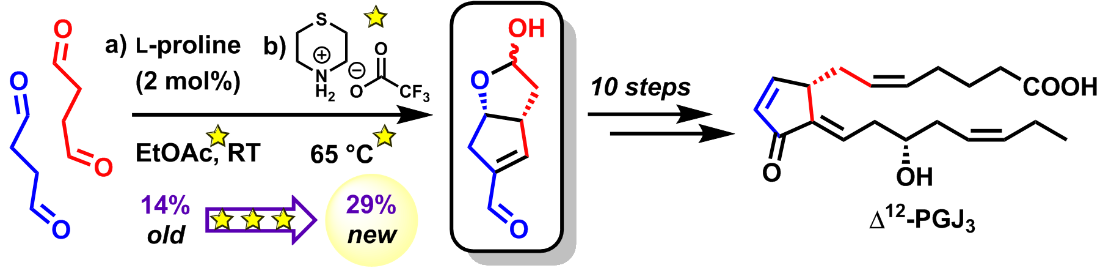
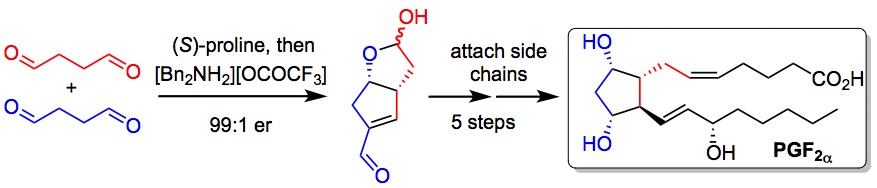
321. Re‐optimization of the Organocatalyzed Double Aldol Domino Process to a Key Enal Intermediate and its Application to the Total Synthesis of Δ¹²‐Prostaglandin J₃
318. (S)‐1‐(Trimethylstannyl)ethyl 2,4,6‐Triisopropylbenzoate and (R)‐1‐(Trimethylstannyl)ethyl 2,4,6‐Triisopropylbenzoate [Review]
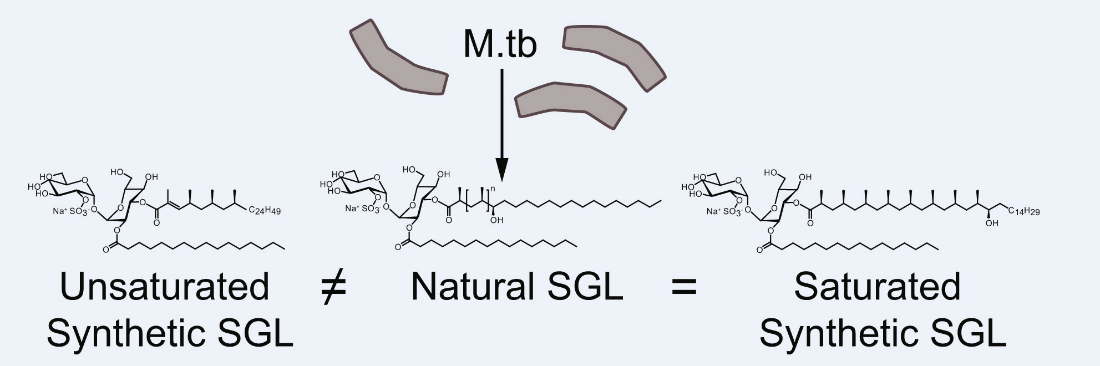
316. CD1b Tetramers Identify T Cells that Recognize Natural and Synthetic Diacylated Sulfoglycolipids from Mycobacterium tuberculosis
315. Visible-Light-Mediated Decarboxylative Radical Additions to Vinyl Boronic Esters: Rapid Access to γ-Amino Boronic Esters
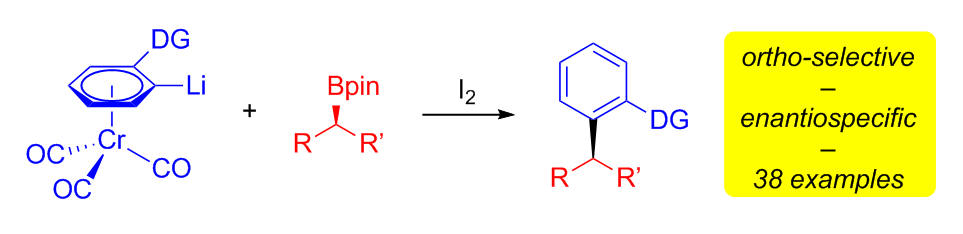
313. ortho-Directing Chromium Arene Complexes as Efficient Mediators for Enantiospecific sp2–sp3 Cross-Coupling Reactions
311. Stereocontrolled synthesis of polypropionate fragments based on a building block assembly strategy using lithiation-borylation methodologies
2017
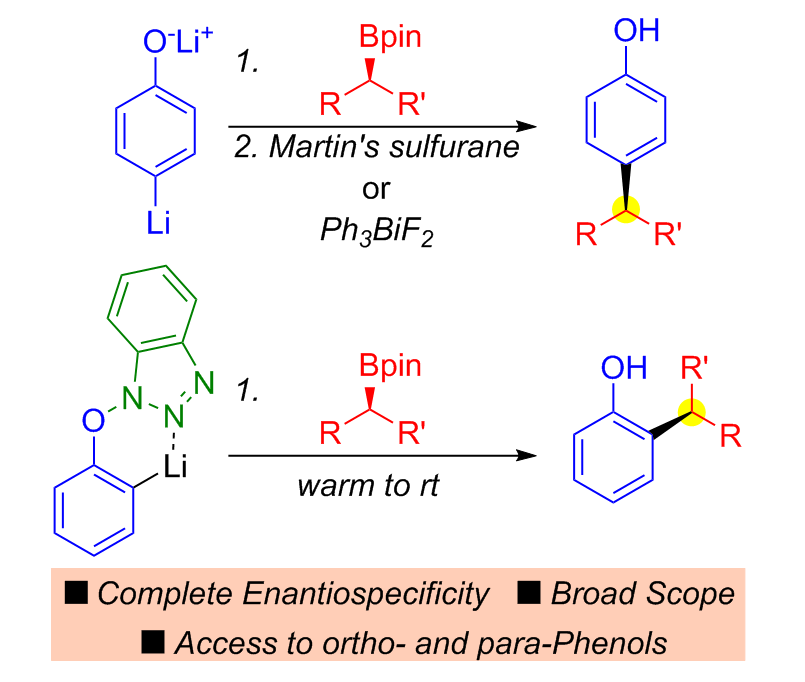
309. Enantiospecific sp2–sp3 Coupling of o- and p-Phenols with Secondary and Tertiary Boronic Esters
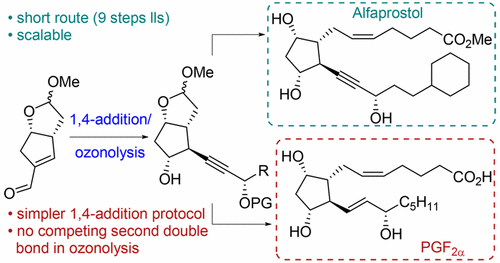
308. Synthesis of Alfaprostol and PGF2α through 1,4-Addition of an Alkyne to an Enal Intermediate as the Key Step
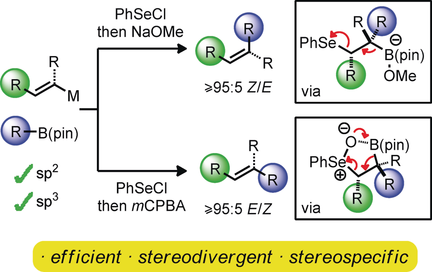
306. Stereospecific Allylic Functionalization: The Reactions of Allylboronate Complexes with Electrophiles
304. α-Sulfinyl Benzoates as Precursors to Li and Mg Carbenoids for the Stereoselective Iterative Homologation of Boronic Esters
303. Asymmetric Synthesis of Tertiary Alcohols and Thiols via Non-Stabilized Tertiary α-Oxy- and α-Thio-Organolithiums
300. Enantioselective Rhodium(III)-Catalyzed Markovnikov Hydroboration of Unactivated Terminal Alkenes
299. Enantiospecific Synthesis of ortho-Substituted Benzylic Boronic Esters by a 1,2-Metalate Rearrangement/1,3-Borotropic Shift Sequence
298. Alkynyl Moiety for Triggering 1,2-Metallate Shifts: Enantiospecific sp2-sp3 Coupling of Boronic Esters with p-Arylacetylenes
295. Conjunctive functionalization of vinyl boronate complexes with electrophiles: a diastereoselective three-component coupling
294. Merging Photoredox with 1,2-Metallate Rearrangements: The Photochemical Alkylation of Vinyl Boronate Complexes
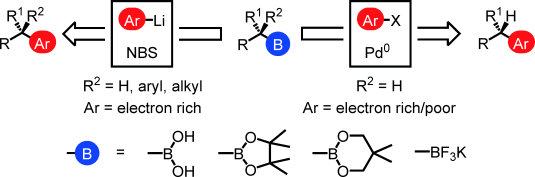
292. Stereospecific functionalizations and transformations of secondary and tertiary boronic esters [Review]
290. Enantiospecific Trifluoromethyl-Radical-Induced Three-Component Coupling of Boronic Esters with Furans
2016
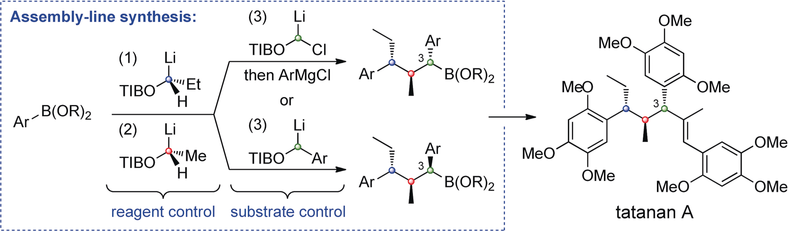
286. Short Enantioselective Total Synthesis of Tatanan A and 3-epi-Tatanan A Using Assembly-Line Synthesis
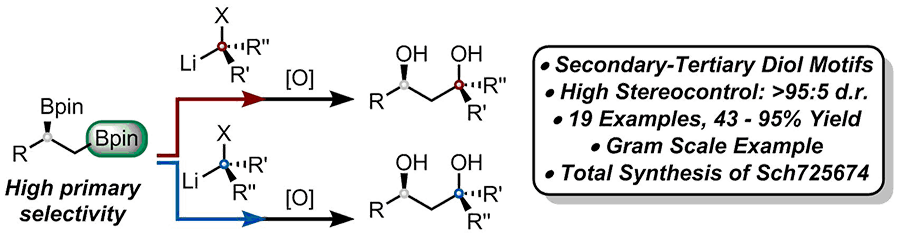
285. Regio- and Stereoselective Homologation of 1,2-Bis(Boronic Esters): Stereocontrolled Synthesis of 1,3-Diols and Sch 725674
284. Development of Enantiospecific Coupling of Secondary and Tertiary Boronic Esters with Aromatic Compounds
283. Synthesis of 3-Aryl-1-aminopropane Derivatives: Lithiation–Borylation–Ring-Opening of Azetidinium Ions
282. Full chirality transfer in the synthesis of hindered tertiary boronic esters under in situ lithiation–borylation conditions
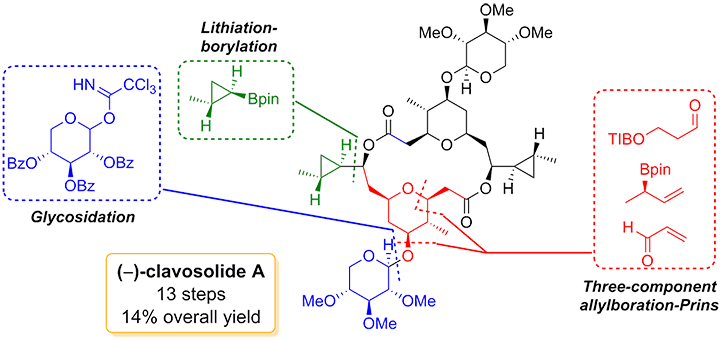
280. Tandem Allylboration–Prins Reaction for the Rapid Construction of Substituted Tetrahydropyrans: Application to the Total Synthesis of (−)-Clavosolide A
279. Activation of the SN2 Reaction by Adjacent π Systems: The Critical Role of Electrostatic Interactions and of Dissociative Character
2015
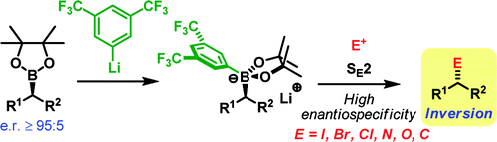
273.Structure and Reactivity of Boron-Ate Complexes Derived from Primary and Secondary Boronic Esters
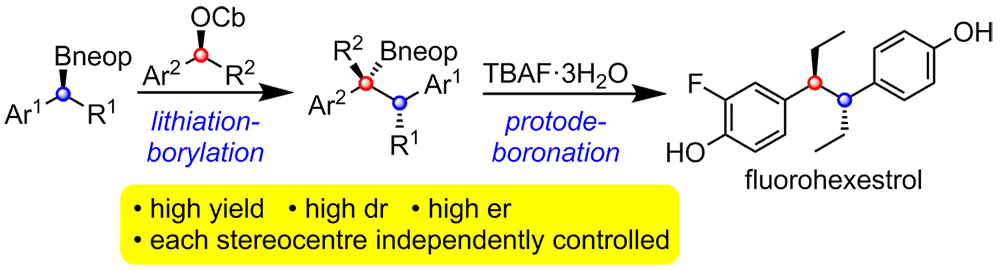
272.Enantioselective installation of adjacent tertiary benzylic stereocentres using lithiation–borylation–protodeboronation methodology. Application to the synthesis of bifluranol and fluorohexestrol
271.Palladium-Catalyzed Reactions of Allylic Boronic Esters with Nucleophiles: Novel Umpolung Reactivity
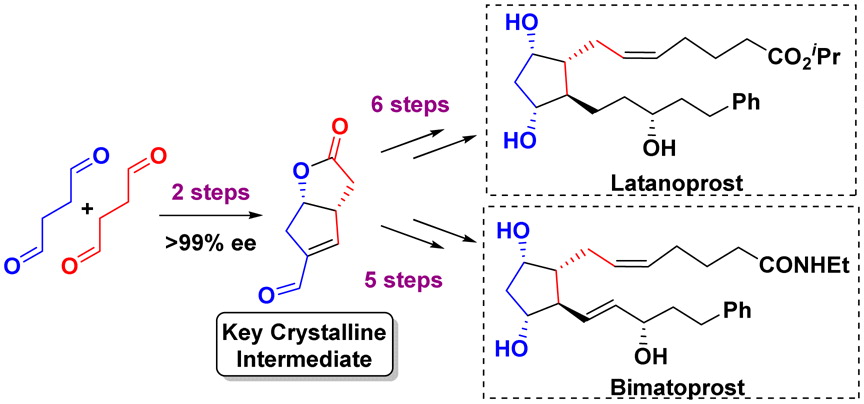
270. Synthesis of Prostaglandin Analogues, Latanoprost and Bimatoprost, Using Organocatalysis via a Key Bicyclic Enal Intermediate
269.Toward Ideality: The Synthesis of (+)-Kalkitoxin and (+)-Hydroxyphthioceranic Acid by Assembly-Line Synthesis
2014
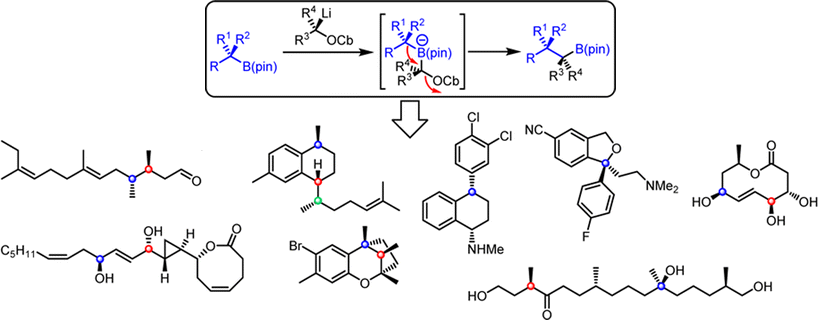
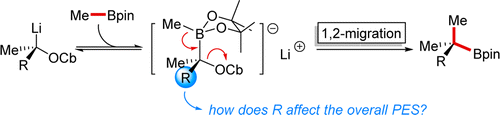
263. Homologation of Boronic Esters with Organolithium Compounds: A Computational Assessment of Mechanism
262.Construction of Multiple, Contiguous Quaternary Stereocenters in Acyclic Molecules by Lithiation-Borylation
261.Synthesis of α-Substituted Vinylsulfonium Salts and Their Application as Annulation Reagents in the Formation of Epoxide- and Cyclopropane-Fused Heterocycles
259.
Highly Diastereoselective and Enantiospecific Allylation of Ketones and
Imines using Borinic Esters: Contiguous Quaternary Stereogenic Centers
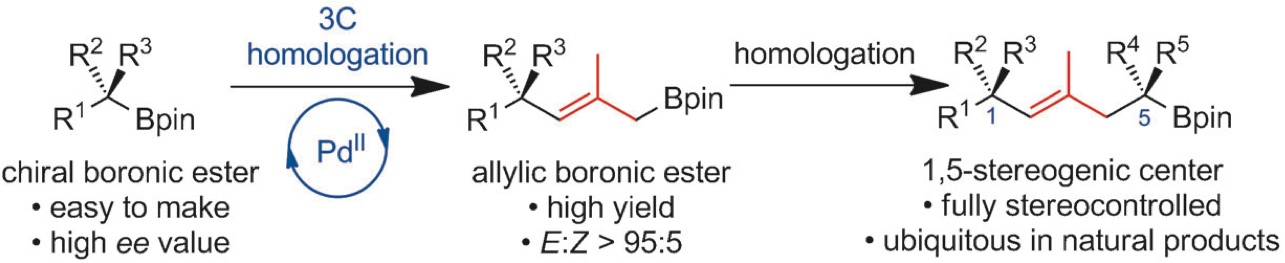
258. Stereocontrolled Synthesis of 1,5-Stereogenic Centers through Three-Carbon Homologation of Boronic Esters.
257. Synthesis of hydroxyphthioceranic acid using a traceless lithiation-borylation-protodeboronation strategy.
255. Highly Selective Allylborations of Aldehydes Using α,α-Disubstituted Allylic Pinacol Boronic Esters.
254.
Stereocontrolled Synthesis of Adjacent Acyclic Quaternary-Tertiary
Motifs and its Application to a Concise Total Synthesis of
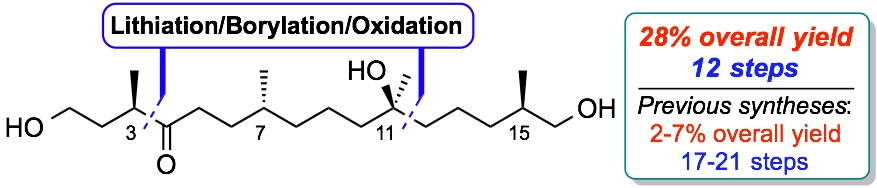
(−)-Filiformin.
253. Short Stereoselective Synthesis of the Phytophthora Universal Mating Hormone α1 Using Lithiation/Borylation Reactions.
252. Stereospecific conversion of alcohols into pinacol boronic esters using lithiation–borylation methodology with pinacolborane.
2013
250. Synthesis of Enantioenriched Tertiary Boronic Esters by the Lithiation/Borylation of Secondary Alkyl Benzoates
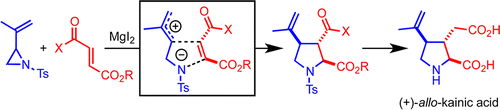
248. Concise Synthesis of (+)-allo-Kainic Acid via MgI2-Mediated Tandem Aziridine Ring Opening–Formal [3 + 2] Cycloaddition
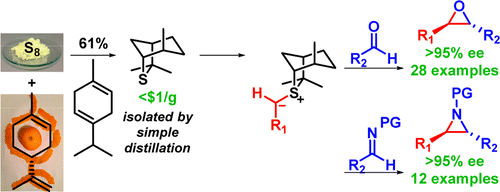
247. Practical and Highly Selective Sulfur Ylide-Mediated Asymmetric Epoxidations and Aziridinations Using a Cheap and Readily Available Chiral Sulfide: Extensive Studies To Map Out Scope, Limitations, and Rationalization of Diastereo- and Enantioselectivities
245. Highly Diastereo-and Enantioselective Allylboration of Aldehydes using α-Substituted Allyl/Crotyl Pinacol Boronic Esters via in situ Generated Borinic Esters
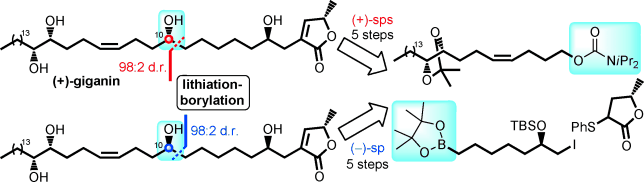
244. Stereoselective Total Synthesis of (+)-Giganin and Its C10 Epimer by Using Late-Stage Lithiation–Borylation Methodology
243. Asymmetric Synthesis of 1-Heteroaryl-1-arylalkyl Tertiary Alcohols and 1-Pyridyl-1-arylethanes by Lithiation–Borylation Methodology
242. One-pot synthesis of 2,3,4,5,6-pentasubstituted tetrahydropyrans using lithiation-borylation, allylation and Prins cyclisation reactions.
2012
239. Diastereoselective Synthesis of CF3‐Substituted, Epoxide-Fused Heterocycles with β‐(Trifluoromethyl)vinylsulfonium Salts
238. Diastereo-divergent Synthesis of Trisubstituted Alkenes through Protodeboronation of Allylic Boronic Esters: Application to the Synthesis of the Californian Red Scale Beetle Pheromone.
237. Enantioselective Synthesis and Cross-coupling of Tertiary Propargylic Boronic Esters Using Lithiation-Borylation of Propargylic Carbamates.
236. Bromoethylsulfonium triflates in stereoselective annulation reactions for the formation of fused bicyclic epoxides and aziridines.
235. Enantioselective Synthesis of (R)-Tolterodine using Lithiation/Borylation Protodeboronation Methodology.
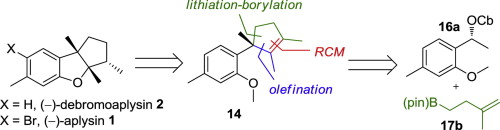
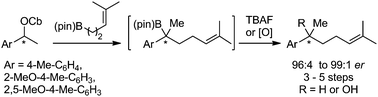
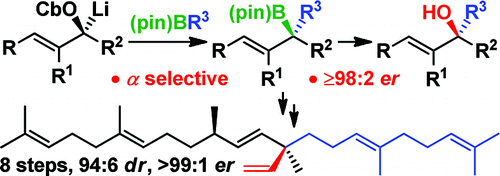
233. The total synthesis of (–)-aplysin via a lithiation-borylation-propenylation sequence.
232. Application of the Lithiation-Borylation Reaction to the Rapid and Enantioselective Synthesis of the Bisabolane Family of Sesquiterpenes.
231. Synthesis of Enantioenriched Tertiary Boronic Esters from Secondary Allylic Carbamates. Application to the Synthesis of C30 Botryococcene.
230. An Efficient Synthesis of Azetidines with (2-Bromoethyl)sulfonium Triflate.
229. Stereocontrolled asymmetric synthesis of syn-E-1,4-diol-2-enes using allyl boronates and its application in the total synthesis of solandelactone F.
228. Synthesis of N-Vinyloxazolidinones and Morpholines from Aminoalcohols and Vinylsulfonium salts: Analysis of the Outcome’s Dependence on N-Protecting group by Nanospray Mass Spectrometry.
2011
227. Highly Enantioselective Synthesis of Tertiary Boronic Esters and their Stereospecific Conversion to other Functional Groups and Quaternary Stereocentres
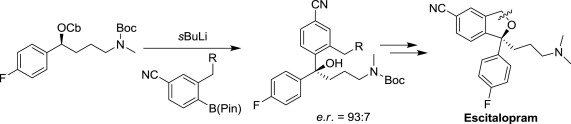
225. Enantioenriched Synthesis of Escitalopram using Lithiation-Borylation Methodology.
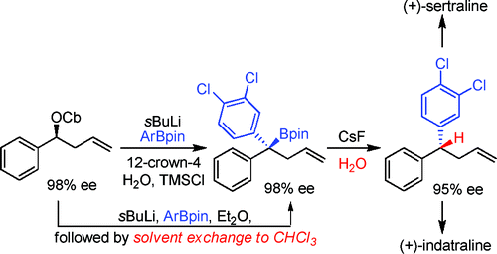
224. Enantioselective Syntheses of (+)-Sertraline and (+) Indatraline using Lithiation/Borylation Protodeboronation Methodology.
223. Ate Complexes of Secondary Boronic Esters as Chiral Organometallic-Type Nucleophiles for Asymmetric Synthesis.
This paper was highlighted in synfacts. pdf
222. Total Synthesis of (+)-Erogorgiaene Using Lithiation–Borylation Methodology, and Stereoselective Synthesis of Each of Its Diastereoisomers.
221. Use of Alkyl 2,4,6-Triisopropylbenzoates in the Asymmetric Homologation of Challenging Boronic Esters.
220. Palladium-Catalyzed Insertion of CO2 into Vinylaziridines: New Route to 5-Vinyloxazolidinones.
218. Sulfinamides as Highly Effective Amine Protecting Groups and their use in the Conversion of Aminoalcohols into Morpholines.
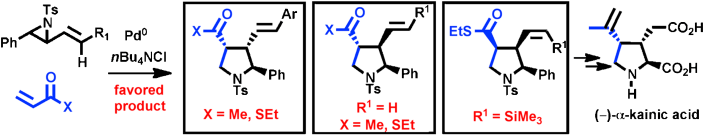
216. Palladium-Mediated Annulation of Vinyl Aziridines with Michael Acceptors. Stereocontrolled Synthesis of Substituted Pyrrolidines and Application to a Formal Synthesis of (–)-α-Kainic Acid.
215. Remote Chiral Induction in Vinyl Sulfonium Salt-Mediated Ring Expansion of Hemiaminals into Epoxide-Fused Azepines
214. Enantioselective Construction of Quaternary Stereogenic Centers from Tertiary Boronic Esters: Methodology and Applications.
213. Asymmetric Synthesis of Tertiary and Quaternary Allyl- and Crotylsilanes via the Borylation of Lithiated Carbamates.
This paper was highlighted in synfacts. (pdf)
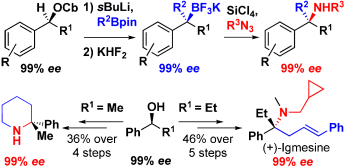
212. Synthesis of Highly Enantioenriched C-Tertiary Amines From Boronic Esters: Application to the Synthesis of Igmesine.
2010
211. Epoxidation and Aziridination of Carbonyl Groups and Imines
210. Protodeboronation of Tertiary Boronic Esters: Asymmetric Synthesis of Tertiary Alkyl Stereogenic Centers.
This paper was highlighted at the Organic Synthesis Portal.
209. Ring-opening of NH-Aziridines with Thiols in Ionic Liquids - Application to the Synthesis of Aminosulfide Catalysts for Asymmetric Epoxidation of Aldehydes.
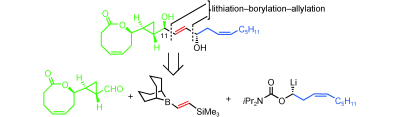
208. Asymmetric Total Synthesis of Solandelactone E: Stereocontrolled Synthesis of the 1,4-diol-2-ene core via Lithiation-Borylation-Allylation Sequence
207. Benzylic Boron Reagents Behaving as Allylic Boron Reagents towards Aldehydes: A New Asymmetric Reaction Leading to Homoallylic Alcohols with Concomitant Dearomatisation
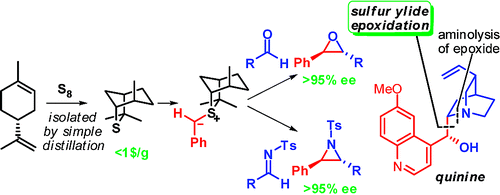
206. Synthesis of Quinine and Quinidine using Sulfur Ylide-mediated Asymmetric Epoxidation as a Key Step.
205. Synthesis of Highly Functionalized 2,5-Disubstituted Pyrrolidines via an Aza-Morita-Baylis-Hillman-type Reaction.
204. Full Chirality Transfer in the Conversion of Secondary Alcohols into Tertiary Boronic Esters and Alcohols Using Lithiation-Borylation Reactions
203. On the Mechanism of Ylide-Mediated Cyclopropanations: Evidence for a Proton Transfer Step and its Effect on Stereoselectivity.
202. Asymmetric Synthesis of Allylsilanes by the Borylation of Lithiated Carbamates: Formal Total Synthesis of (-)-Decarestrictine D.
Angew. Chem. Int. Ed., 2010, 49, 4264-4268. doi | pdf
Highlighted in Chemistry World: Totally Synthetic
Highlighted in Chemistry World: Totally Synthetic
201. A Novel Asymmetric Azaspirocyclisation using a Morita Baylis Hillman-type Reaction.
200. Application of the Lithiation-Borylation Reaction to the Preparation of Enantioenriched Allylic Boron Reagents and Subsequent In Situ Conversion into 1,2,4-Trisubstituted Homoallylic Alcohols with Complete Control over All Elements of Stereochemistry.
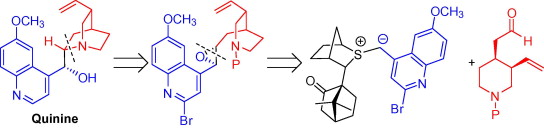
199. Practical and Highly Selective Sulfur Ylide-Mediated Asymmetric Epoxidations and Aziridinations Using a Cheap and Readily Available Chiral Sulfide. Application to the Total Synthesis of Quinine and Quinidine.
J. Am. Chem. Soc., 2010, 132, 1828-1830. doi | pdf
Presented in Chemistry By Design
Highlighted in Chemistry World
Presented in Chemistry By Design
Highlighted in Chemistry World
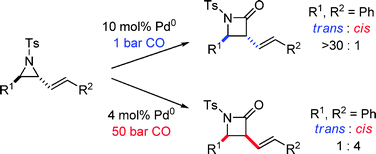
198. Stereoeselective Synthesis of trans-β-Lactams by Palladium-Catalysed Carbonylation of Vinyl Aziridnes. (pdf, 1.0Mb)
Chem. Commun., 2010, 46, 267-269.
2009
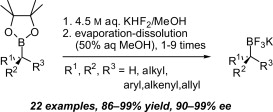
197. Homologation and Alkylation of Boronic Esters and Boranes by 1,2-Metallate Rearrangement of Boron Ate Complexes
196. Improved Method for the Conversion of Pinacolboronic Esters into Trifluoroborate Salts. Facile Synthesis of Chiral Secondary and Tertiary Trifluoroborates. (pdf)
Tetrahedron, 2009, 65, 9956-9960.
195. New Uses for Old Building Blocks
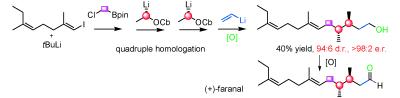
194. Stereocontrolled Synthesis of Carbon Chains Bearing Contiguous Methyl Groups by Iterative Boronic Ester Homologations. Application to the Total Synthesis of (+)-Faranal. (pdf)
Angew. Chem. Int. Ed., 2009, 48, 6317-6319.
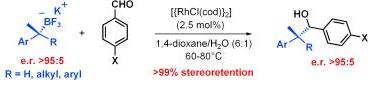
193. Complete Stereoretention in the Rhodium-Catalyzed 1,2-Addition of Chiral Secondary and Tertiary Alkyl Potassium Trifluoroborate Salts to Aldehydes. (pdf)
Angew. Chem. Int. Ed., 2009, 48, 6289-6292.
192. The Fate of the tert-Butylsulfinyl Auxiliary After Acid-Promoted Cleavage - a Method for Recycling t-BuSONH2. (pdf)
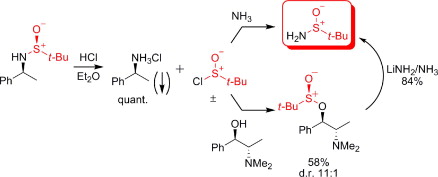
191. Asymmetric Hydroboration of 1,1-Disubstituted Alkenes. (pdf, 1.0Mb)
Angew. Chem. Int. Ed., 2009, 48, 1896-1898.
190. Stereocontrolled Synthesis of β-Amino Alcohols from Lithiated Aziridines and Boronic Esters. (pdf)
Angew. Chem. Int. Ed., 2009, 48, 1149-1152.
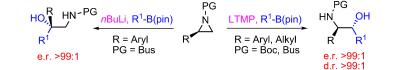
189. Bromoethylsulfonium salt - a More Effective Annulation Agent for the Synthesis of 6- and 7-Membered 1,4-Diheterocyclic Compounds. (pdf)
Org. Lett., 2009, 11, 257-260.
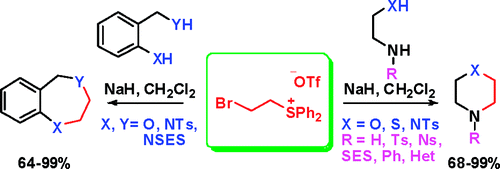
188. Homologation of Boronic Esters with Lithiated Epoxides for the Stereocontrolled Synthesis of 1,2 and 1,3-Diols, and 1,2,4-Triols. (pdf)
Org. Lett. 2009, 11, 165-168.
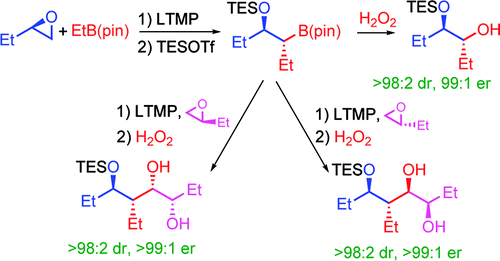
2008
187. Synthesis of Epoxides by Carbonyl Epoxidation
186. Highly Enantioselective Conversion of Secondary Alcohols into Tertiary Alcohols with Broad Scope. (pdf, 3.3Mb)
Nature, 2008, 456, 778-782.
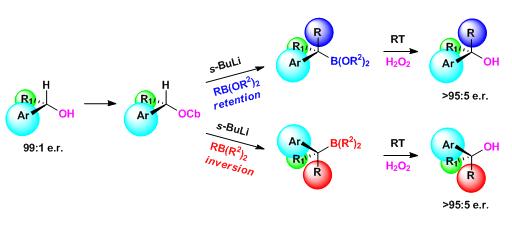
185. Synthesis and Application of Easily Recyclable Thiomorpholines for use in Sulfur Ylide-mediated Asymmetric Epoxidation of Aldehydes.
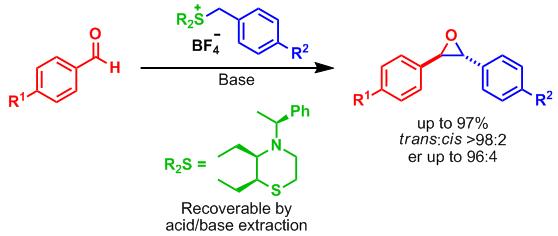
184. Sulfur Ylide Mediated Three-Component Aziridination and Epoxidation Reactions Using Vinyl Sulfonium Salts Thre-Component Aziridination and Epoxidation Reaction. (pdf)
Synlett, 2008, 2191-2195.
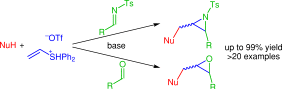
183. Direct Synthesis of Functionalized Allylic Boronic Esters From Allylic Alcohols and Inexpensive Reagents and Catalysts. (pdf)
Synthesis, 2008, 2293-2297.
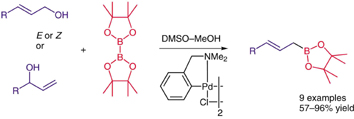
182. Novel Annulation Reaction for the Synthesis of Morpholines, Thiomorpholines and Piperazines from β-Heteroatom Amino Compounds and Vinyl Sulfonium Salts. (pdf)
Angew. Chem. Int. Ed., 2008, 47, 3784-3786.
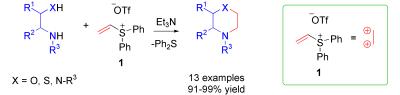
181. Epoxy-Annulations by Reactions of α-Amino Ketones with Vinyl Sulfonium Salts. Reagent Versus Substrate Control and Kinetic Resolution. (pdf)
Org. Lett., 2008, 10, 1501-1504.
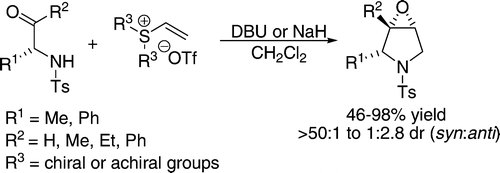
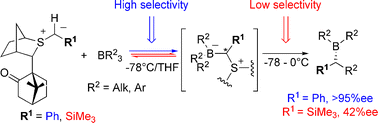
180. Reactions of Silyl-Stabilised Sulfur Ylides with Organoboranes: Enantioselectivity, Mechanism, and Understanding. (pdf, 3.4Mb)
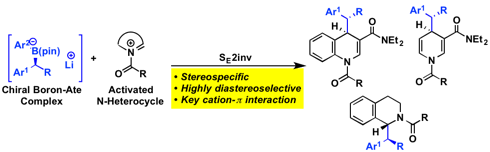
Org. Biomol. Chem., 2008, 6, 1185-1189.
179. Asymmetric Lithiation-Substitution of Amines Involving Rearrangement of Borates. (pdf)
Org. Lett., 2008, 10, 141-143.
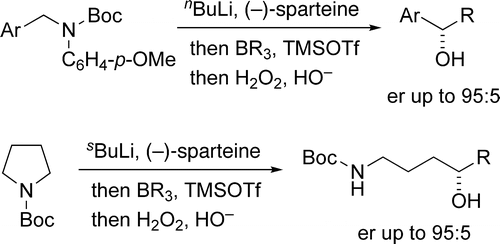
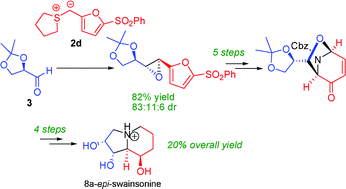
178. Application of Furyl-Stabilized Sulfur Ylides to a Concise Synthesis of 8a-epi-Swainsonine. (pdf)
Chem. Commun., 2008, 120-122.
2007
177. Chalcogenides as Organocatalysts
176. Ylide Based Reactions
174. Mechanism of the Morita-Baylis-Hillman Reaction: A Computational Investigation.
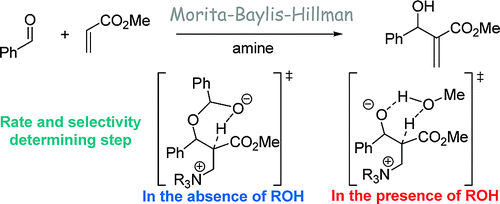
173. Asymmetric Sulfur Ylide Reactions with Boranes: Scope and Limitations, Mechanism and Understanding.
J. Am. Chem. Soc., 2007, 129, 14632-14639.
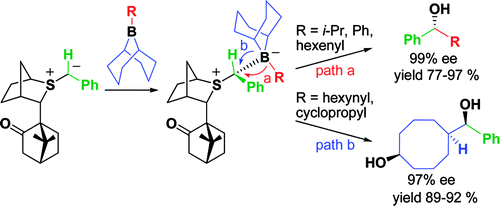
172. A New Manifold for the Morita Reaction: Diene Synthesis from Simple Aldehydes and Acrylates/Acrylonitrile Mediated by Phosphines.
Chem. Commun., 2007, 4128-4130.
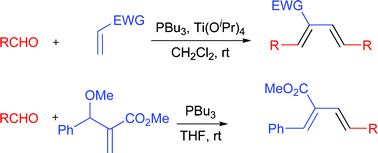
171. Lithiated Carbamates: Chiral Carbenoids for Iterative Homologation of Boranes and Boronic Esters.
170. Aminals as Substrates for Sulfur Ylides: A Synthesis of Functionalised Aziridines and N-Heterocycles.
Org. Lett., 2007, 9, 2099-2102.
169. New Reaction of Cyclic Aminal with Michael Acceptors Leading to β-Amido-α,β-unstaurated Carbonyl Compounds through a Morita-Baylis-Hillman Type Reaction. Enantiocontrol and Applications in Synthesis.
168. Asymmetric Synthesis of α-Substituted Allylic Boranes and their Application in Synthesis of iso-Agatharesinol.
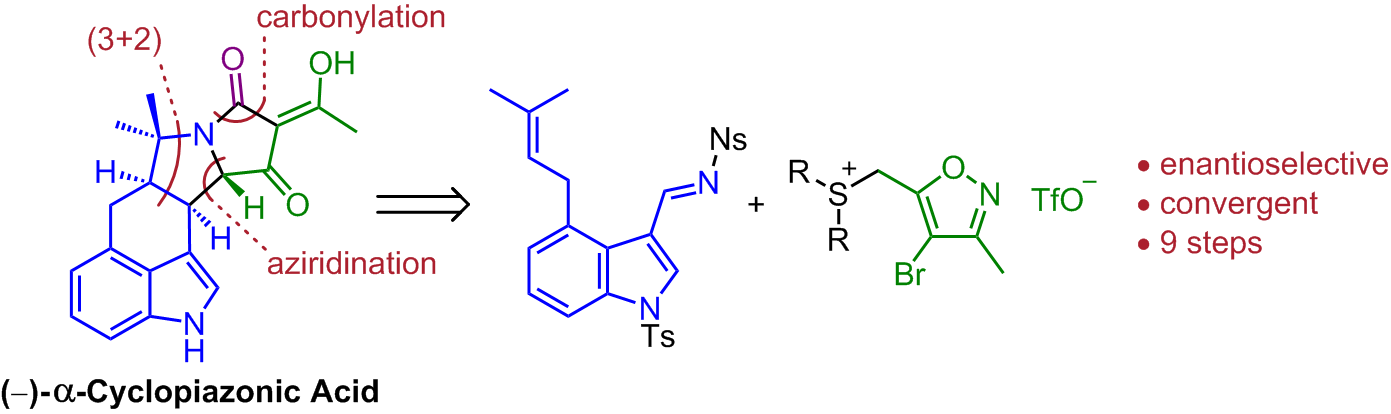
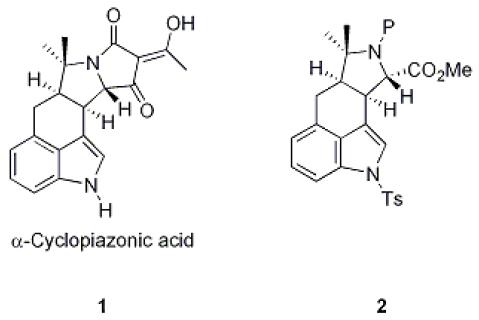
167. Studies Towards a Biomimetic Synthesis of α-Cyclopiazonic Acid: Synthesis of 5-Substituted Isoxazole-4-carboxylic Esters.
Arkivoc, 2007, part V, 139-151.
2006
166. Rearrangements of Organozinc Compounds
165. Towards an Understanding of the Factors Responsible for the 1,2-Migration of Alkyl Groups in Borate Complexes
164. Catalityc Asymmetric Sulfur Ylide Mediated Epoxidation of Carbonyl Compounds
163. Asymmetric synthesis of epoxides and aziridines from aldehydes and imines
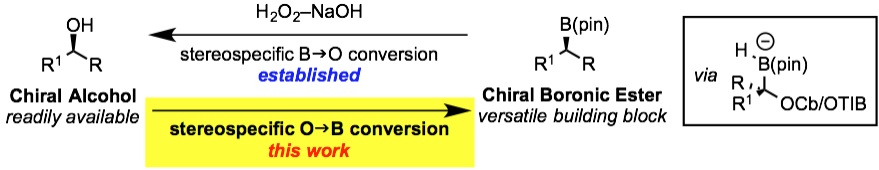
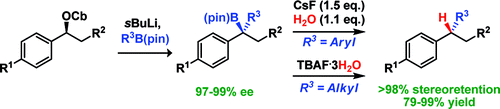
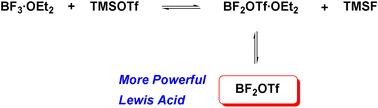
160. BF3·OEt2 and TMSOTf: A Synergistic Combination of Lewis Acids
Chem. Commun., 2006, 4434-4436.
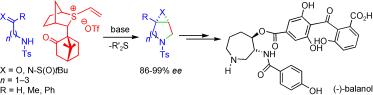
159. The Use of Vinyl Sulfonium Salts in the Stereocontrolled Asymmetric Synthesis of Epoxide- and Aziridine-Fused Heterocycles: Application to the Synthesis of (-)Balanol
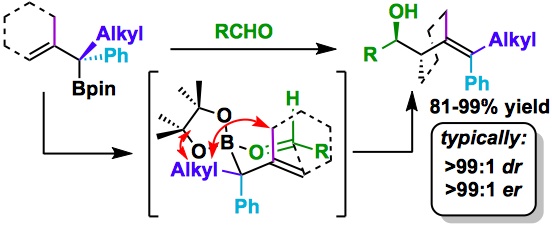
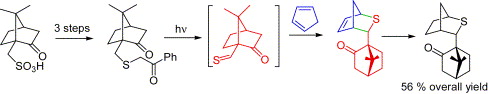
158. A Practical Synthesis of a [2,2,1] Bicyclic Chiral Sulfide for Asymmetric Transformations
157. Novel, Readily-Synthesised, Chiral Sulfides as Reagents for Asymmetric Epoxidation
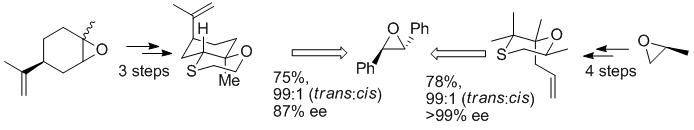
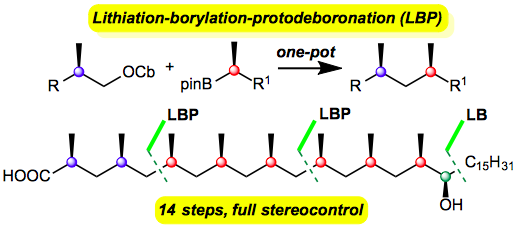
156. Hemiaminals as Substrates for Sulfur Ylides: Direct Asymmetric Syntheses of Functionalised Pyrrolidines and Piperidines
Chem. Commun., 2006, 2156-2158.
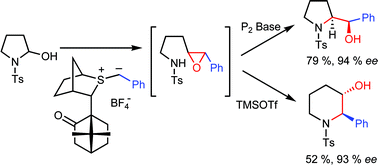
155. Ligand Induced Control of C-H Versus C-C Insertion Reactions of Rh Carbenes
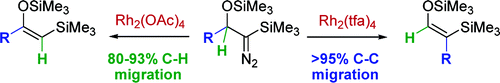
154. Reactivity and Selectivity in the Wittig Reaction: A Computational Study

153. Highly Enantioselective Synthesis of Glycidic Amides Using Camphor-Derived Sulfonium Salts. Mechanism and Applications in Synthesis
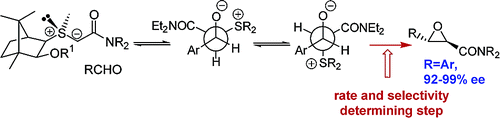
152. Is phenyl a Good Migrating Group in the Rearrangement of Organoborates Generated from Sulfur Ylides?
Chem. Commun., 2006, 741-743.
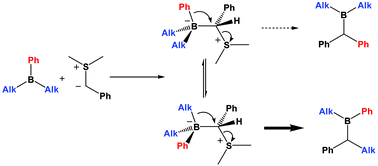
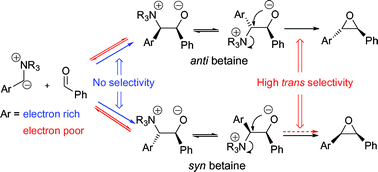
151. Delineation of the Factors Governing Reactivity and Selectivity in Epoxide Formation from Ammonium Ylides and Aldehydes
Org. Biomol. Chem., 2006, 4, 621-623.
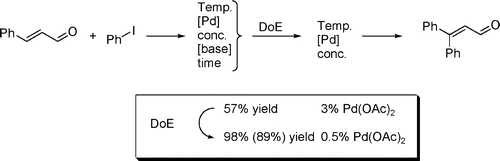
150. Optimization of the Mizoroki-Heck Reaction using Design of Experiment (DoE)
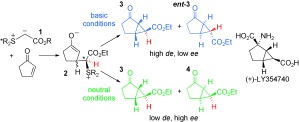
149. Asymmetric Sulfonium Ylide Mediated Cyclopropanation: Stereocontrolled Synthesis of (+)-LY354740
2005
148. The Use of Tosylhydrazone Salts as a Safer Alternative to Handling Diazo Compounds and their Applications in Organic Synthesis
147. α-Substituted Sulfur Ylides
145. On the Origin of High E Selectivity in the Wittig Reaction of Stabilized Ylides: Importance of Dipole-Dipole Interactions
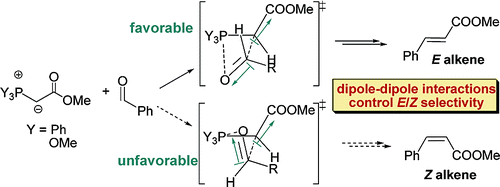
144. Enantioselective α-Arylation of Cyclohexanones with Diaryl Iodonium Salts: Application to the Synthesis of (-)-Epibatidine
143. Highly regioselective and Diastereoselective Epoxidation of Allylic amines with Oxone
142. Highly Diastereoselective Diels-Alder Reactions of Baylis-Hillman Adducts
141. On the Importance of Leaving Group Ability in Reactions of Ammonium, Oxonium, Phosphonium and Sulfonium Ylides
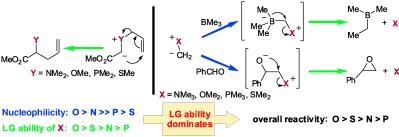
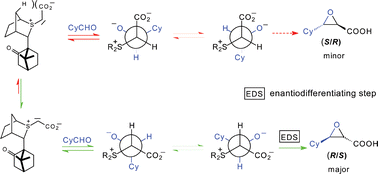
140. Carboxylate-Stabilised Sulfur Ylides (Thetin Salts) in Asymmetric Epoxidation for the Synthesis of Glycidic Acids. Mechanism and Implications
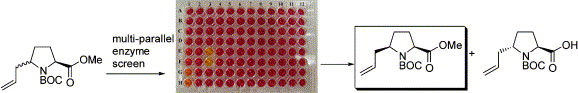
139. Separation of Pyrrolidine Allylation Products by Diastereoselective Enzymatic Ester Hydrolysis
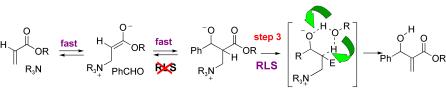
137. Re-evaluation of the Mechanism of the Baylis-Hillman Reaction - Implications for Asymmetric Catalysis
136. Simple Preparation of Trans-epoxides via Ylide Intermediates
Pre-2005
135. Sulfur Ylides
134. Catalytic Asymmetric Sulfur Ylide-Mediated Synthesis of epoxidation of Carbonyl Compounds: Scope, Selectivity and Applications in Synthesis
133. Lithiation and Reactions of Stilbene oxides: Synthetic Utility
132. Application of Sulfur Ylide Mediated Epoxidations in the Asymmetric Synthesis of β-Hydroxy-δ-Lactones. Synthesis of a Mevinic Acid Analogue and (+)-Prelactone B
131. Asymmetric Synthesis of Avenaciolide via Cascade Palladium Catalysed Cyclisation-Carbonylation of Bromodienes
130. A Concise Asymmetric Route to the Bridged Bicyclic Tropane Alkaloid Ferruginine using Enyne Ring Closing Metathesis
129. Effect of Sulfide Structure on Enantioselectivity in Catalytic Asymmetric Epoxidation of Aldehydes; Mechanistic Insights and Implications
128. The Complexity of Catalysis: Origins of Enantio- and Diastereocontrol in Sulfur Ylide Mediated Epoxidation Reactions
127. Profile of V. K. Aggarwal
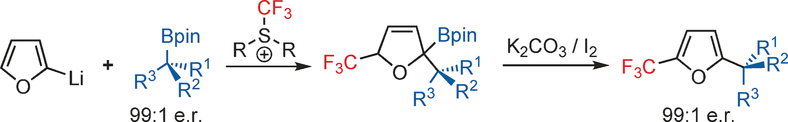
126. Catalytic Asymmetric Nazarov Reactions Promoted by Chiral Lewis Acid Complexes
125. Highly Diastereoselective Simmons-Smith Cyclopropanation of Allylic Amines
124. Diastereoselective Synthesis of Cyclopropane Amino Acids Using Diazo Compounds Generated In Situ
123. Asymmetric Sulfur Ylide Mediated Aziridination: Application in the Synthesis of the Side Chain of Taxol
122. Tandem Formation and [2,3] Rearrangement of Methylene Ammonium Ylides Derived from Amines and the Simmons-Smith Reagent
121. Palladium catalysed cyclisation-carbonylation of enynes to give cyclic γ,δ-unsaturated acids
120. Highly Diastereoselective 1,3-Dipolar Cycloaddition Reactions of trans-2-Methylene-1,3-dithiolane 1,3-dioxide with 3-Oxidopyiridinium and 3-Oxidopyrylium Betaines: a Route to the Tropane Skeleton
119. Sulfur Ylide Mediated Synthesis of Highly Functionalised and Trisubstituted Epoxides with High Enantioselectivity. Application to the Synthesis of CDP 840
118. A New Protocol for the in situ Generation of Aromatic, Heteroaromatic and Unsaturated Diazocompounds and its Application in Catalytic and Asymmetric Epoxidation of Carbonyl Compounds. Extensive Studies to map out Scope and Limitations and Rationalization of Diastereo- and Enantioselectivities
117. Highly Enantioselective Oxidations of Ketene Dithioacetals: Formation of Trans Bis-sulfoxides without Contamination from Meso Isomers
116. A Novel One-pot Method for the Preparation of Pyrazoles by 1,3-Dipolar Cycloadditions of Diazo Compounds Generated In Situ
115. Generation of Phosphoranes Derived from Phosphites. A New Class of Phosphorus Ylides Leading to High E Selectivity with Semi-Stabilizing Groups in Wittig Olefinations
114. New insights in the Mechanism of Amine Catalysed Epoxidation: Dual Role of Protonated Ammonium Salts as Both Phase Transfer Catalysts and Activators of Oxone
113. The use of Enantiomerically Pure Ketene Dithioacetal bis-Sulfoxides in Highly Diastereoselective Intramolecular Nitrone Cycloadditions. Application in the Total Synthesis of α-Amino Acids, (-)-Cispentacin and the First Asymmetric Synthesis of 4-Amino-pyrrolidine-3-carboxylic acid
112. Correlation Between pKa and Reactivity of Quinuclidine-based Catalysts in the Baylis-Hillman Reaction - Discovery of Quinuclidine as Optimum Catalyst Leading to Substantial Enhancement of Scope
111. Extension of Ring Closing Metathesis Methodology to the Synthesis of Carbocyclic Methyl and Silyl Enol Ethers
110. Ketene Claisen Rearrangement of Camphor-derived 1,3-oxathianes: complete control of tertiary and quaternary stereogenic centres
109. Application of the Chiral Acyl Anion Equivalent, trans-1,3-Dioxide, to an Asymmetric Synthesis of (R)-Salbutamol
108. A palladium catalysed cyclisation-carbonylation of bromodienes: control in carbonylation over facile beta-hydride elimination
107. Sulphur ylide-mediated stereoselective synthesis of a stable ferrocenyl epoxide
106. Unraveling the mechanism of epoxide formation from sulfur ylides and aldehydes
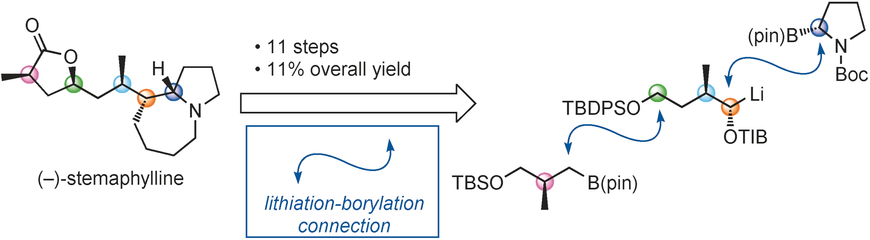
105. Synthesis of epoxides from aldehydes and tosylhydrazone salts catalysed by triphenylarsine: complete trans selectivity for all combinations of coupling partners
104. Unexpected side reactions of imidazolium-based ionic liquids in the base-catalysed Baylis-Hillman reaction
103. Highly enantioselective Darzens reaction of a camphor-derived sulfonium amide to give glycidic amides and their applications in synthesis
102. A new method for the preparation of silyl enol ethers from carbonyl compounds and (Trimethylsilyl)diazomethane in a regiospecific and highly stereoselective manner
101. Epoxidation of alkenes by amine catalyst precursors: Implication of aminium ion and radical cation intermediates (vol 122, pg 8317, 2000)
100. Synthesis of new, highly hindered C-2-symmetric trans (2S,5S)- disubstituted pyrrolidines
99. Highly diastereoselective aziridination of imines with trimethylsilyldiazomethane. Subsequent silyl substitution with electrophiles, ring opening, and metalation of C- silylaziridines - A cornucopia of highly selective transformations
98. Highly diastereoselective nitrone cycloaddition onto a chiral ketene equivalent: Asymmetric synthesis of cispentacin
97. The use of enantiomerically pure N-sulfinimines in asymmetric Baylis-Hillman reactions
96. Rate Acceleration of the Baylis-Hillman Reaction in Polar Solvents (Water and Formamide). Dominant Role of Hydrogen Bonding not Hydrophobic Effects are Implicated
95. Developments in the Simmons-Smith Mediated Epoxidation Reaction
94. Additions of stabilised and semi-stabilised sulfur ylides to tosyl protected imines: are they under kinetic or thermodynamic control?
93. Synthesis and evaluation of a broad range of chiral sulfides for asymmetric sulfur ylide epoxidation of aldehydes
92. Catalytic Cyclopropanation of Alkenes using Diazo Compounds Generated In Situ. A novel route to 2-Arylcyclopropylamines
91. A novel procedure for the synthesis of aziridines: application of Simmons-Smith reagents to aziridination
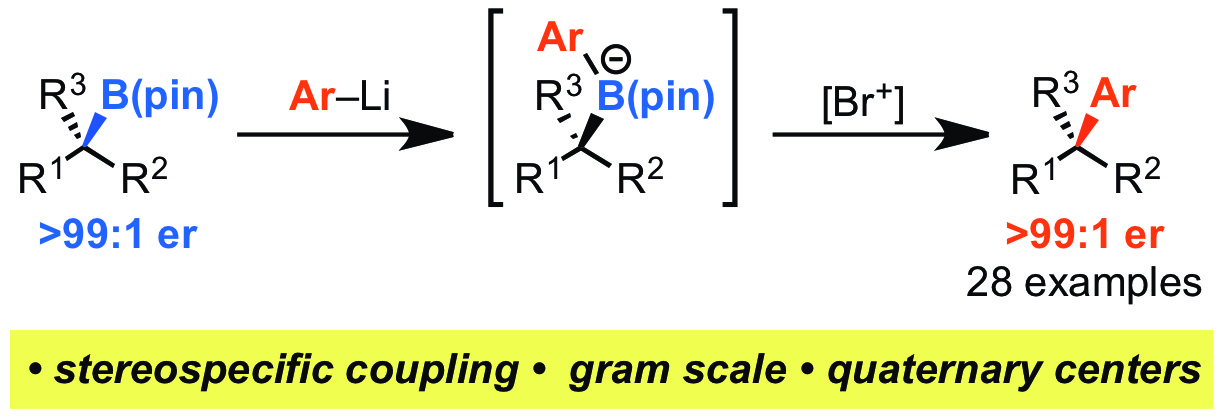
90. Scope and limitations in sulfur ylide mediated catalytic asymmetric aziridination of imines: use of phenyldiazomethane, diazoesters and diazoacetamides
89. Application of Chiral Sulfides to Catalytic Asymmetric Aziridination and Cyclopropanation with in situ Generation of the Diazocompound
88. Catalytic Asymmetric Synthesis of Epoxides from Aldehydes using Sulfur Ylides with in situ Generation of Diazocompounds
87. Profile of Professor Varinder K. Aggarwal (Corday Morgan Award)
86. A simple, user-friendly process for the homologation of aldehydes using tosylhydrazone salts
85. Highly Selective Aziridination of Imines Using Trimethylsilyldiazomethane and Applications of C-Silylaziridines in Synthesis
84. Amidine-promoted addition of chloroform to carbonyl compounds
83. Epoxidation of alkenes by amine catalyst precursors: Implication of aminium ion and radical cation intermediates
82. Catalytic cyclopropanation of electron deficient alkenes mediated by chiral and achiral sulfides: scope and limitations in reactions involving phenyldiazomethane and ethyl diazoacetate
81. Stereochemical control in the synthesis of tetrahydrofurans by cyclisation of diols with [1,2]-phenylsulfanyl migration
80. Copper(II)-bisoxazoline-catalysed asymmetric Diels-Alder reactions of alpha-thioacrylates
79. Epoxidation of Aldehydes and Enones
78. Sulfur Ylide Mediated Catalytic Asymmetric Epoxidation and Aziridination
76. Diastereomerically pure spirocyclic bis-sulfinyl oxiranes and their application to the asymmetric synthesis of alpha-amino amides
75. A formal synthesis of (+)-pyripyropene A using a biomimetic epoxy- olefin cyclisation: effect of epoxy alcohol/ether on cyclisation efficiency
74. [2,3]-Sigmatropic Rearrangement of Allylic Sulfur Ylides Derived from Trimethylsilyldiazomethane (TMSD)
73. Enantioselective deprotonation of 4-tert-butylcyclohexanone by conformationally constrained chiral lithium amide bases
72. Superior amine catalysts for the Baylis-Hillman reaction: the use of DBU and its implications
71. An improved resolution of 2-methyl piperidine and its use in the synthesis of homochiral trans-2,6-dialkyl piperidines
70. A Formal Asymmetric Synthesis of (+)-Anatoxin-a Using an Enantioselective Deprotonation Strategy on an Eight Membered Ring
69. A formal synthesis of (+)-pyripyropene A using a biomimetic epoxy- olefin cyclisation
68. The effects of different ester and ketal protecting groups on the reactivity and selectivity of tartrate-derived silylketene acetals
67. Direct Asymmetric Epoxidation of Aldehydes using Catalytic Amounts of Enantiomerically Pure Sulfides
66. Sc(OTf)3, an efficient catalyst for addition of allyltrimethylsilane to aldehydes; Chemoselective addition to aldehydes in presence of ketones and in situ acylation (3 component coupling)
65. Studies on the asymmetric oxidation of ester derivatives of 1,3- dithiane-2-carboxylates. Asymmetric synthesis of trans-1,3-dithiane 1,3-dioxide
64. Metal- and ligand-accelerated catalysis of the Baylis-Hillman reaction
63. Highly diastereoselective epoxidation of ketene dithioacetal dioxides: A new approach to the asymmetric synthesis of alpha-amino amides
62. Catalytic Sylfur Ylide Reactions: Use of Diazoacetamides for the Diastereoselective Synthesis of Glycidic Amides
61. Catalytic asymmetric Diels-Alder reactions of alpha-thioacrylates for the preparation of norbornenone
60. (1R,3R)-2-methylene-1,3-dithiolane 1,3-dioxide: a highly reactive and highly selective chiral ketene equivalent in cycloaddition reactions with a broad range of dienes
59. Catalytic asymmetric epoxidation of aldehydes. Optimization, mechanism, and discovery of stereoelectronic control involving a combination of anomeric and Cieplak effects in sulfur ylide epoxidations with chiral 1,3-oxathianes
58. Sc(OTf)3, an efficient catalyst for formation and deprotection of geminal diacetates (acylals); Chemoselective protection of aldehydes in presence of ketones
57. Bifunctional catalysts for catalytic asymmetric sulfur ylide epoxidation of carbonyl compounds
56. 1,3-Dipolar cycloaddition reactions of trans-2-methylene-1,3- dithiolane 1,3-dioxide with nitrones
55. Camphor Derived 1,4-Oxathianes for Carbonyl Epoxidation
54. Catalytic Asymmetric Epoxidation and Aziridination Mediated by Sulfur Ylides. Evolution of a Project
Synlett, 1998, 329-336.
53. Scandium trifluoromethanesulfonate, an efficient catalyst for the intermolecular carbonyl-ene reaction and the intramolecular cyclisation of citronellal
52. Asymmetric Ylide Reactions: Epoxidation, Cyclopropanation, Aziridination, Olefination, and Rearrangement
51. A novel procedure for the synthesis of epoxides: Application of Simmons-Smith reagents toward epoxidation
50. Trans-1,3-Dithiane-1,3-Dioxide; a Chiral Acyl Anion Equivalent. Enantioselective Synthesis of ?-Hydroxy-Carboxylic Acids, Esters, Amides and Ketones
49. Catalytic Asymmetric Cyclopropanation of Electron Deficient Alkenes Mediated By Chiral Sulfides.
48. Dimethylsulfonium 9-Fluorenide - Solution Structure and Dynamic Behaviour of a Semi-Stabilised Sulfonium Ylide
47. Asymmetric total synthesis of (+)-carbocyclic uracil polyoxin C
46. Scope and Limitations in Palladium Catalysed Substitution Reactions of Unsaturated Fused Lactones
45. Trifluoromethanesulfonic Acid, an Efficient Catalyst for the Hetero Diels-Alder Reaction and an Improved Synthesis of Mefrasol
44. Addition of Benzyl Sulfonium Ylides to Aldehydes and Ketones. Are they under Kinetic or Thermodynamic Control?
43. Electronic Conference on Organometallic Chemistry: Catalytic Asymmetric Epoxidation
42. Seminars in Organic Synthesis: Asymmetric Epoxidation, XXII Summer School, Gargnano, Società Chimica Italiana
41. Catalytic and Asymmetric Aziridination Using Sulfur Ylides
40. Stereselective Epoxidation of bis-Sulfinyl Alkenes and Applications to the Asymmetric Synthesis of ?-Substituted Carboxylic Acids
39. Anion Reactions of Trans-1,3-Dithiolane-1,3-Dioxide with Aldehydes and Comparison with Trans-1,3-Dithiane-1,3-Dioxide
38. Direct Measurement of the Anomeric Effect in Sulfoxides: Conformational Analysis and X-Ray crystal Structures of 2-Halo-1,3-Dithiane-trans-1,3-Dioxide
37. 2-Halo-1,3-Dithiane-1,3-Dioxide : a Diastereoselective Carbonyl Anion Equivalent in Reactions with Aldehydes
36. First Examples of Metal and Ligand Accelerated Catalysis of the Baylis Hillman Reaction
35. Novel Catalytic and Asymmetric Process for Aziridination Mediated by Sulfur Ylides
34. Direct Asymmetric Epoxidation of Aldehydes Using Catalytic Amounts of Enantiomerically Pure Sulfides
33. First Synthesis and X-ray Crystal Structure of 1,2-(1,1'-Ferrocenediyl)ethene
32. A Novel Catalytic Cycle For The Synthesis Of Epoxides Using Sulfur Ylides
31. Scandium Trifluoromethanesulfonate, a Novel Catalyst for the Addition of Allyltrimethylsilane to Aldehydes
30. Palladium-Catalyzed Substitution Of Unsaturated Lactones - Application to the Synthesis Of Carbocyclic Polyoxins and Nikkomycins
29. Catalytic Asymmetric Synthesis of Epoxides Mediated by Chiral Iminium Salts
28. The Use Of Chiral Sulfides In Catalytic Asymmetric Epoxidation
27. (1R,3R)-2-Methylene-1,3-Dithiolane 1,3-Dioxide - a Highly Reactive and Highly Selective Chiral Ketene Equivalent
26. Anion reactions of 1,3-dithiane 1,3-dioxide with carbonyl-compounds - high diastereoselectivity with aromatic-aldehydes under conditions of equilibrium control
25. Complexes Containing a Lewis-Acid and Bronsted Acid For the Catalytic Asymmetric Diels-Alder Reaction
24. A Novel Catalytic Cycle For the Synthesis Of Epoxides Using Sulfur Ylides - Application to Base Sensitive Aldehydes
23. A novel catalytic cycle for the synthesis of epoxides using sulfur ylides, and application to the synthesis of cyclopropanes and aziridines V. K. Aggarwal; H. Abdel-Rahman; A. Thompson; B. Mattison; R. V. H. Jones Phosphorus Sulfur 1995, 283-292.
22. Synthesis of Sulfonium Salts by Sulfide Alkylation; an Alternative Approach
21. Asymmetric-Synthesis and Cycloaddition Chemistry Of Trans-2- Methylene-1,3-Dithiolane 1,3-Dioxide
20. Studies on the oxidation of 1,3-dithiane and 5,5-disubstituted analogues including X-ray crystal structure, equlibration studies and pKa measurements on selected oxides
19. Stereoselective Addition Reactions of Lithiated 2-Chloro-1,3-Dithiane-1,3-Dioxide to Aldehydes
18. Trans-1,3-dithiane-1,3-dioxide, a new chiral acyl anion equivalent for the preparation of masked activated acids - application to the synthesis of alpha-hydroxy acid-derivatives
17. Novel catalytic cycle for the synthesis of epoxides from aldehydes and sulfur ylides mediated by catalytic quantities of sulfides and Rh2(OAc)4
16. Asymmetric epoxidation using chiral sulfur ylides
15. The First Synthesis of the Novel 2,8-Dioxobicyclo[3,2,1]octane Ring System - a Key Feature of the Squalestatins
14. Enantioselective Transformations and Racemisation Studies of Heteroatom Substituted Organolithium Compounds
13. Chiral Ketene Equivalents for use in Asymmetric Synthesis
12. Chiral, Bisfunctionalisation of Substrates: a Powerful Strategy for the Asymmetric Synthesis of C2 Symmetric Compounds and its Application to the Synthesis of Enantiomerically Pure Trans-1,3-dithiane-1,3-dioxide 1
11. Trans-Dioxides of Cyclic Ketene Thioacetals: Highly Selective Chiral Ketene Equivalents
10. Highly Stereoselective Addition Reactions of Metallated Trans-1,3-dithiane-1,3-dioxide to Aldehydes
9. Synthesis, X-ray Crystal Structure, Equilibration Studies, and Anion Chemistry of Trans-1,3-dithiane-1,3-dioxide
8. Transformation of Cyclic a-Phenylthio Aldehydes by Stereoselective Aldol Reactions and Phenylthio Migration into Spirocyclic Lactones and Ethers, and E-Allylic Alcohols with 1,4-Related Chiral Centres
7. Trans-1,3-Dithiane-1,3-dioxide as a Potential Chiral Acyl Anion Equivalent
6. Additions of Aldehydes to Metallated Trans-1,3-dithiane-S,S-dioxide Under Conditions of Kinetic and Thermodynamic Control
5. Stereochemical Control in the Synthesis of Tetrahydrofurans by Cyclisation of Diols with Phenylthio Migration
4. A Review of S. G. Warren's Recent Work
3. Synthesis of Compounds with 1,4-Related Chiral Centres by Phenylthio Migration: Syn and Anti-E-2,4 Dimethylhex-3-ene-1,5-Diol
2. Allyl Sulphide Synthesis by Phenylthio Migration: Relationship between Stereochemistry of Starting Material and Regioselectivity of Double Bond Formation
1. Phenylthio (PhS) Migration in the Stereocontrolled Synthesis of Allylic Alcohols with 1,4-Related Chiral Centres