 Pulsed Laser Deposition of Carbon-Containing Targets
Pulsed Laser Deposition of Carbon-Containing Targets
 PLD in Vacuum
PLD in Vacuum
Pulsed Laser Deposition (PLD) has now been proven to be a versatile and reliable technique for the deposition of a wide variety of materials and compounds ranging from high-temperature superconductors, ferroelectrics, metals, polymers, ceramics through to diamond-like-carbon thin films. The experimental set-up and procedure is relatively simple compared to the extreme complexity of the ablation process itself, since this involves laser-solid interaction at the target, plasma formation and directional transport of material to the substrate upon which deposition takes place.
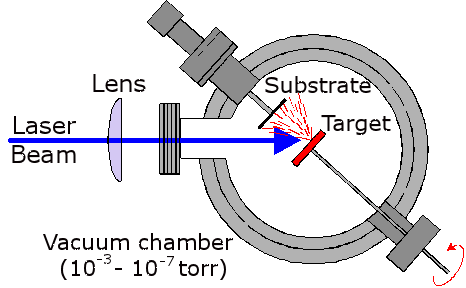
Essentially, a high power laser is focused onto a substrate in vacuum (see diagram, right). The extreme energy density at the focus causes the breakage of many chemical bonds within the target, and a few surface layers are ejected at high kinetic energy in the form of an ablation 'plume'. The components of these layers, which can be in the form of ions, electrons, atoms, radicals or clusters, travel ballistically across the vacuum chamber until they strike a substrate material (normally silicon). The high impact energies (typically 100 eV) causes the particles to stick to the surface, and be compressed, forming a dense continuous film. Subsequent laser pulses gradually build up the thickness of the film from a few atomic layers to microns.
The PLD process has numerous parameters which are chosen so as to be optimum for the specific application. For example: wavelength, target material, fluence (energy/unit area), vacuum conditions, presence/pressure of an ambient gas, be it inert or reactive, angle of incidence of laser beam relative to target surface and substrate temperature.
Here at Bristol, the main thrust of ablation work was the production of thin films of diamond-like carbon (DLC), and was done in parallel with our activities on making DLC fims by Rf plasma deposition. The title DLC covers a wide range of materials often produced from very different deposition techniques, giving materials that possess an extremely wide range of physical properties due to varying amounts of hydrogen and sp2/sp3-bonded carbon. The detailed composition and properties of such films depends sensitively on the exact parameters employed during their preparation.
Ablation of graphite under vacuum leads to the condensation of high energy carbon atoms, ions and clusters onto the substrate. The high energy of the incident particles provides the driving force to convert from sp2 (graphite) to sp3-bonded (diamond) carbon. These films are extremely smooth (on the nm scale), hydrogen free and are termed amorphous carbon (a-C), whilst still being under the general heading of DLC. Such films with high sp3:sp2 ratios are often referred to as tetrahedral amorphous carbon (ta-C).
PLD from graphite in the presence of hydrogen gas offers one route for the deposition of hydrogenated amorphous carbon films (a-C:H). Ablation of polymeric targets such as polycarbonate or poly(methyl methacrylate (PMMA, perspex) under vacuum or in the presence of hydrogen provides another. Hydrogenated a-C:H films offer similar properties to a-C films, whilst allowing the variation of optical band gap simply by varying the hydrogen content within the films.
These films display many of the same attractive physical properties as polycrystalline diamond, but have the advantage that deposition is possible at room temperature. This is the key advantage over other techniques such as CVD which require the substrate to be maintained at elevated temperatures (~1000 K). Low temperature deposition allows growth onto temperature-sensitive substrates, such as glass and plastics. Since DLC is a mixture of sp2 and sp3 bonded carbon, it is highly defective, which leads to it possessing a low electron affinity and excellent electron emission characteristics. This has caused great interest for potential use in cold cathode emission devices. The low temperature deposition makes this technology compatible with delicate microelectronic circuitry and silica glass substrates. Deposition using a mask allows patterned a-C and a-C:H films to be produced, which is one of the prerequisites to using them in many devices.
Little is known about the formation, propagation and chemical composition of laser plasmas and the majority of the work undertaken at Bristol concentrated on trying to understand the processes involved in the transport of material from the target to the substrate and how these affect film characteristics.
Film Deposition
Above right is a schematic plan view of the ablation chamber, and a photo of it below. An excimer ArF laser (Lambda Physik, Compex 201, 193 nm) with a typical pulse energy of 2-300 mJ was focused using a fused-silica lens (focal length = 27 cm) onto the target mounted in a stainless-steel vacuum chamber maintained at ~10-6 torr. The area of the focal volume has been estimated by stylus profilometry to be ~ 1 × 0.4 mm2.
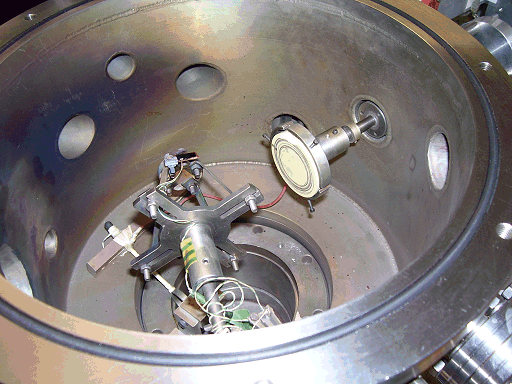
The target, made from materials such as graphite, PMMA or ZnO, takes the form of a 50 mm-diameter disk mounted on a shaft attached via a double 'O' ring seal to a stepper motor. This enables the target to be rotated during ablation to prevent deep crater formation, allowing longer deposition times and reducing the number of large particulates being sputtered from the target and being incorporated into the films. The laser is incident at an angle of 45°.
Films are deposited on various substrates, including single-crystal silicon, quartz and NaCl, each ~1 cm2 in area, or single-crystal diamond (1 mm2). The substrate holder allows the target-substrate distance to be varied between 30 and 60 mm by means of a micrometer and bellows arrangement. The substrate can be heated to a temperature of ~800 °C by using a 30 W CO2 laser which is defocused onto the back of the substrate.
Wavelength Dispersed Optical Emission Spectroscopy
The focal volume adjacent to the target, and the ablation plume, are both clearly visible via their accompanying optical emission. The light emitted from the expanding plume is imaged through a lens onto a quartz fibre optic bundle (Oriel) located behind a lens/iris combination and an appropriately positioned quartz observation port mounted in the top flange of the chamber. This optical arrangement restricts the viewing zone in the vicinity of the plume to a column estimated at ~2 mm in diameter and is translatable to provide spatial resolved emission spectra. An example of the expanding emission plume can be seen below.
 |
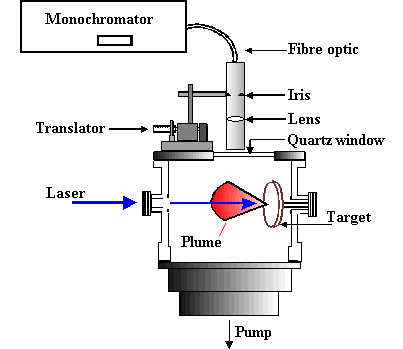 |
| Animation of the expanding ablation plume made from multiple still images. | OES set-up to view the plume. |
The other end of the fibre bundle butts up against the entrance slit of either of two monochromators. One is a 12.5 cm monochromator (Oriel), equipped with a 600 lines/mm ruled grating and an Instaspec IV CCD array detector, which is capable of providing low-resolution dispersed emission spectra covering a wide (~300 nm) range of wavelengths from just a single laser shot, the signal to noise of which can be enhanced by summing for many laser shots. The second is a 0.5 m Spex 1870 monochromator equipped with a 2400 lines/mm holographic grating and entrance and exit slits whose widths are user selectable. Light emerging from the exit slit of this monochromator is detected with a red-sensitive photomultiplier tube (PMT). Higher resolution emission spectra were obtained by scanning the monochromator with the slit widths set narrow and passing the PMT output via a boxcar and a V/F converter to a PC.
PLD from Liquids - Production of Novel NanocrystalsWe also studied the ablation of carbon targets submerged in a liquid medium, such as water or cyclohexane. This technique is called Liquid Phase Pulsed Laser Ablation (LP-PLA), and in this system, the ablation plume interacts with the liquid molecules and forms cavitation bubbles, which then collapse and produce extremely high local temperatures and pressures, but on the sub-micron or nm scale. We have used this system successfully to make nanocrystals of diamond as well as nanocrystals of novel materials, such as carbon nitride. Depending on the ablation conditions, the nanoparticles self-assembled into a range of unusual architectures, such as nanoleaves, nanorods, and branched, flowerlike shapes. |
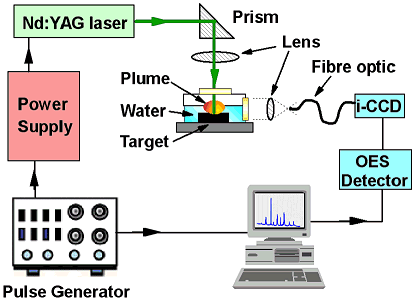 Schematic diagram of the liquid phase pulsed laser ablation set-up. |
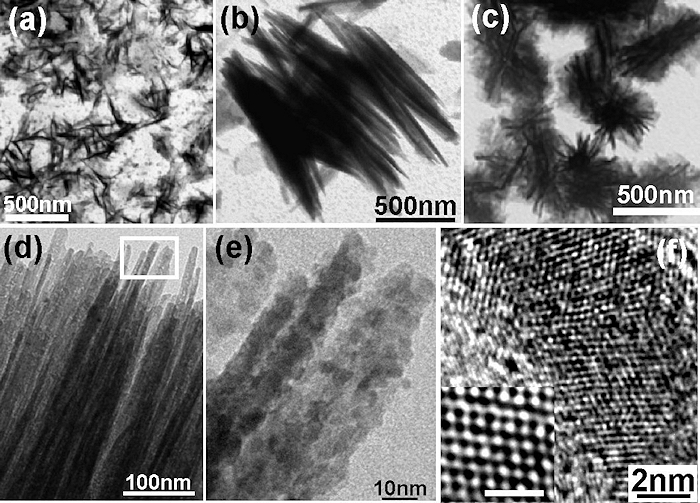
Left: TEM images of the range of nanostructures obtained by LP-PLA in 25% ammonia solution:
(a) isolated carbon nitride nanorods, (b) Branched nanorods, (c) highly branched flowerlike architectures,
(d) Rod-like structures showing straight, long and sharp tips, (e) Enlarged image of the region at the top of the nanorods indicated by the open box in (d),
(f) HR-TEM image of a single nanorod, the inset shows the atomic arrangement.
Related Papers
- W.N. Wang, N.A. Fox, P.W. May, M.P. Knapper, G. Meaden, P.G. Partridge, M.N.R. Ashfold, J.W. Steeds, L.P. Hayward and G.D. Pitt "Laser Raman Studies of Polycrystalline and Amorphic Diamond Films", Physica Stat. Solido., 154 (1995) 255.
- S.E. Johnson, M.N.R. Ashfold, M.P. Knapper, R.J. Lade, K.N. Rosser, N.A. Fox and W.N. Wang, "Production and Characterisation of Amorphic Diamond Films Produced by Laser Ablation of Graphite", Diamond Relat. Mater. 6 (1997) 569.
- F. Claeyssens, R.J. Lade, K.N. Rosser and M.N.R. Ashfold, "Investigations of the plume accompanying pulsed ultraviolet laser ablation of graphite in vacuum" J. Appl. Phys., 89 (2001) 697-709.
- G.M. Fuge, C.J. Rennick, S.R.J. Pearce, P.W. May and M.N.R. Ashfold, "Structural characterisation of CNx thin films deposited by pulsed laser ablation", Diamond Relat. Mater. 12 (2003) 1049-54
- M.N.R. Ashfold , F. Claeyssens , G.M. Fuge and S.J. Henley, "Pulsed laser ablation and deposition of thin films", Chem. Soc. Rev., 33 (2004) 23-31.
- G.M. Fuge, P.W. May, K.N. Rosser, S.R.J. Pearce, M.N.R. Ashfold, "Laser Raman and X-ray photoelectron spectroscopy of phosphorus containing diamond-like carbon films grown by pulsed laser ablation methods", Diamond Relat. Mater. 13 (2004) 1442-48
- S.R.J. Pearce, S.J. Henley, F. Claeyssens, P.W. May, K.R. Hallam, J.A. Smith, K.N. Rosser, "Production of nanocrystalline diamond by laser ablation at the solid/liquid interface", Diamond Relat. Mater. 13 (2004) 661-665.
- L. Yang, P.W. May, L. Yin, R. Brown, T. Scott, "Direct growth of highly organized crystalline carbon nitride from liquid phase pulsed laser ablation", Chem. Mater. 18 (2006) 5058-5064.
- L. Yang, P.W. May, L. Yin, T.B. Scott, "Growth of self-assembled ZnO nanoleaf from aqueous solution by pulsed laser ablation", Nanotechnol. 18 (2007) 215602.
- L. Yang, P.W. May, L. Yin, J.A. Smith, K.N. Rosser, "Ultra fine Carbon Nitride Nanocrystals Synthesized by Laser Ablation in Liquid Solution", J. Nanoparticle Res. 9 (2007) 1181-1185.
- L. Yang, P.W. May, L. Yin, J.A. Smith, K.N. Rosser, "Growth of diamond nanocrystals by pulsed laser ablation of graphite in liquid", Diamond Relat. Mater. 16 (2007) 725-729.
- L. Yang, P.W. May, L. Yin, "Self-assembled hierarchical architectures by liquid-phase pulsed laser ablation", Chapter 8 in the book Nanotechnology Research Developments, ed. R. Jiménez-Contreras (Nova Science Publishers, New York, USA, 2008) , pp.295-314.
- K. Honglertkongsakul, P.W. May, B. Paosawatyanyong, "Electrical and optical properties of diamond-like carbon films deposited by pulsed laser ablation", Diamond Relat. Mater. 19 (2010) 999-1002. [doi: 10.1016/j.diamond.2010.03.007]
- K. Honglertkongsakul, P.W. May, B. Paosawatyanyong, "Effect of temperature on sulfur-doped diamond-like carbon films deposited by pulsed laser ablation", Diamond Relat. Mater. 20 (2011) 1218-1221. [doi: 10.1016/j.diamond.2011.07.002]
- K.D.G.I. Jayawardena, Y.Y. Tan, J. Fryar, H. Shiozawa, S.R.P. Silva, S.J. Henley, G.M. Fuge, B.S. Truscott and M.N.R. Ashfold, "Highly conductive nanoclustered carbon:nickel films grown by pulsed laser deposition", Carbon 49 (2011) 3781-8. [doi: 10.1016/j.carbon.2011.05.012]
- J.N. Hart, P.W. May, N.L. Allan, K.R. Hallam, F. Claeyssens, G.M. Fuge, M. Ruda, P.J. Heard, "Towards new binary compounds: Synthesis of amorphous phosphorus carbide by pulsed laser deposition", J. Solid State Chem. 198 (2013) 466-474. [doi: 10.1016/j.jssc.2012.11.008]
