Adjvants for Agrochemicals

Nicola Nugent (previous student, left the group in 2003)
Introduction
Surfactants are commonly used in pesticide formulations, as bioenhancing adjuvants, to disperse or solubilise the active ingredient. It is important to understand the way in which these surfactants interact with the active ingredient. Techniques such as NMR self-diffusion and solvent relaxation as well as dynamic light scattering are useful tools in studying surfactant - active ingredient interactions.
Solvent Relaxation NMR
The Technique
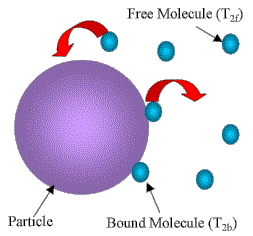
- NMR can discriminate between highly mobile (liquid like) and immobile (solid like) protons on the basis of magnetic relaxation times.
- In this case, we measure the spin-spin relaxation time, T2, of solvent molecules, which is directly related to their mobility.
- Bulk water molecules: T2 of the order of a couple of seconds.
- Water molecules bound at an interface: shorter T2 because of much more efficient relaxation due to their constrained motion.
For the case of rapid exchange between interface and bulk, this results in an effective relaxation rate that is the weighted sum of the two.
Results
This technique has been used to investigate the adsorption of a non-ionic surfactant onto a styrene-acrylic copolymer latex. (Polymer latex particles can be considered a reasonable model for the active ingredient). Some initial results are presented below.
 Graph 1: Relaxation Rate as a function of solids concentration (constant surfactant concentration).
Graph 1: Relaxation Rate as a function of solids concentration (constant surfactant concentration).
 Graph 2: Relaxation Rate as a function of surfactant concentration (constant particle concentration).
Graph 2: Relaxation Rate as a function of surfactant concentration (constant particle concentration).
The relaxation rate of the solvent increases linearly with particle concentration (graph1). This is due to an increase in available surface area and consequently and increase in the proportion of bound solvent molecules. Addition of surfactant leads to a further enhancement of relaxation rate, indicating an increase in bound water molecules.
Varying surfactant concentration while keeping the particle concentration constant allows measurement of a pseudo adsorption isotherm (graph 2). The data shows a gradual increase in relaxation rate as surfactant concentration is increased, followed by a plateau when full surface coverage is reached. This indicates a Langmuir-like adsorption isotherm.
Photon Correlation Spectroscopy has been used to study the same system, and has revealed some interesting adsorption behaviour which is hoped can be fully explained by future Small Angle X-ray and Neutron Scattering (SAXS and SANS) experiments.
The above techniques have also been used to study the interactions between the surfactant and the actual active ingredient.
Future work
- SANS experiments on the latex-surfactant system to investigate the adsorbed layer.
- Conventional isotherms of the latex-surfactant system.
- Further NMR experiments involving the real active ingredient.
- Pulsed Field Gradient NMR experiments to investigate the surfactant micelles (and liquid crystal phases at higher concentration).
This project is funded by the EPSRC and Syngenta