 Thermionic Devices
Thermionic Devices
Diamond is a very unusual material, in that if its surface is terminated with a (sub)monolayer of certain species, such as H, Si and most metals, it exhibits so-called negative electron affinity (NEA). This means that electrons can be emitted from its surface without having to overcome an energy barrier, such that the energy (temperature or voltage) required to eject the electrons is significantly reduced, while the number of electrons emitted (the emission current) is greatly increased.
 Thermionic emission
Thermionic emission
One method by which the electrons can be extracted from the diamond surface is simply by heating the diamond to high temperature in vacuum. This 'boiling' off of electrons is known as 'thermionic emission', and due to the NEA surface, occurs at a much lower temperature in diamond than in other materials (such as tungsten), which usually require temperatures in excess of 1500 °C. Thermionic emission begins for hydrogenated diamond at only ~500 °C, and by ~900 °C the emission current density often exceeds 1 mA cm-2. Unfortunately, the relatively low adsorption energy of ~ −4 eV for H onto diamond means that these surfaces remain stable to only ~600–800 °C, after which the H atoms start to desorb leaving a bare surface without an NEA leading to a significant decrease in emission current.
A lot of work has been done by the Bristol group and others, therefore, to develop diamond surface terminations with large NEA values, and hence low workfunctions, which retain their NEA properties while remaining stable at high temperatures. Most of our work on alternative termination species has involved depositing monolayers or sub-monolayers of electropositive metals (M), such as Li, Mg, Na, Ti, Al, and Sc, onto the bare or oxidised diamond surface.
Indeed, we have recently found that scandium-terminated diamond has the highest NEA for a metal adsorbed onto bare diamond measured to date (–1.45 and –1.13 eV on diamond (100) and (111) surfaces, respectively), as well as being thermally stable up to 900 °C. Si and Ge terminations have also been investigated experimentally and theoretically, and look very promising. We are continuing to study different metal adsorbate schemes with an aim to greatly improve the electron emission efficiency.
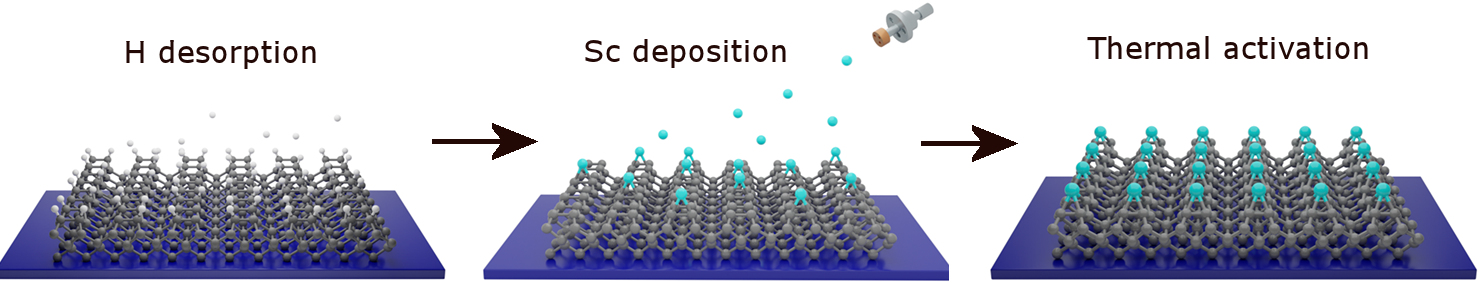
The process for deposition of a submonolayer of Sc onto hydrogenated diamond. The diamond is first heated in high vacum to desorb the H, the scandium is evaporated from a high-purity Sc rod for a known time to produce around 1/4 monolayer. Finally, the surface is annealed at ~900 °C to chemically bond the Sc to the C surface.
Thermionic Energy Converters
 Due to its NEA surface, diamond has been proposed as a candidate for energy generation using a thermionic energy converter (TEC). In a TEC, electrons emitted from a hot cathode (the emitter) travel across a high-vacuum gap and are captured by a cooler anode (the collector). The difference in work function between the anode and cathode creates a potential difference between the electrodes. Connecting the two electrodes completes the circuit, and permits electrons to flow from the collector back to the emitter, driving an electric current through a load. Thus, heat can be converted directly into electricity within a solid-state device that has no moving parts. Ideally, the heating of the emitter would be obtained in the form of infrared light from the sun, which would be collected using a solar-tracking array and focused onto the back of the emitter. A solar TEC, such as this might provide a complementary technology to standard photovoltaic (PV) solar cells for renewable energy generation. If the thermionic emission temperature can be reduced to 200-300 °C, it may also be possibe to use these TECs for heat-scavenging applications, where the waste heat from engines, turbines, furnaces or incinerators could be captured and converted to useful electricity.
Due to its NEA surface, diamond has been proposed as a candidate for energy generation using a thermionic energy converter (TEC). In a TEC, electrons emitted from a hot cathode (the emitter) travel across a high-vacuum gap and are captured by a cooler anode (the collector). The difference in work function between the anode and cathode creates a potential difference between the electrodes. Connecting the two electrodes completes the circuit, and permits electrons to flow from the collector back to the emitter, driving an electric current through a load. Thus, heat can be converted directly into electricity within a solid-state device that has no moving parts. Ideally, the heating of the emitter would be obtained in the form of infrared light from the sun, which would be collected using a solar-tracking array and focused onto the back of the emitter. A solar TEC, such as this might provide a complementary technology to standard photovoltaic (PV) solar cells for renewable energy generation. If the thermionic emission temperature can be reduced to 200-300 °C, it may also be possibe to use these TECs for heat-scavenging applications, where the waste heat from engines, turbines, furnaces or incinerators could be captured and converted to useful electricity.
Related Papers
For our papers about electron emission from variously terminated diamond surfaces (not necessariy for thermionic applications) please also see the electron-emission page.
- H. Dominguez-Andrade, A. Croot, G. Wan, J.A. Smith, N.A. Fox, "Characterisation of thermionic emission current with a laser-heated system", Rev. Sci. Instrum. 90 (2019) 045110.
- H. Dominguez-Andrade, J. Anaya, A. Croot, M. Cattelan, D.J. Twitchen, M. Kuball, N.A. Fox, "Correlating Thermionic Emission with Specific Surface Reconstructions in a <100> Hydrogenated Single-Crystal Diamond", ACS Appl. Mater. Interfaces 12 (2020) 26534.
- M.C. James, F. Fogarty, R. Zulkharnay, N.A. Fox, P.W. May, "A Review of Surface Functionalisation of Diamond for Thermionic Emission Applications", Carbon 171 (2020) 532.
- Michael C. James, PhD, "Aluminium and Oxygen Termination of Diamond for Thermionic Applications", February 2020.
- Yuet Mun Gary Wan, PhD, "Characterization of Electron Emission From Diamond Surfaces For Energy Conversion Devices", January 2021.
- R. Zulkharnay, N.L. Allan and P.W. May, "Ab initio study of negative electron affinity on the scandium-terminated diamond (100) surface for electron emission devices", Carbon 196 (2022) 176-185. [doi: 10.1016/j.carbon.2022.04.067].
- R. Zulkharnay, P.W. May, "Experimental Evidence for Large Negative Electron Affinity from Scandium-terminated Diamond", J. Mater. Chem. A 11, (2023) 13432-13445. [doi: 10.1039/D2TA09199B]
- R. Zulkharnay, N.A. Fox, P.W. May, "Enhanced Electron Emission Performance and Air-Surface Stability in ScO-terminated Diamond for Thermionic Energy Converters", Small 20 (2024) 2405408. [doi: 10.1002/smll.202405408]
